Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Dactylitis is a key and frequent clinical manifestation of psoriatic arthritis (PsA)1 associated with significant disease burden and impaired function.2–3 Randomized controlled trials designed to evaluate the impact of biologics on resolution of dactylitis are limited.3 We report results of a post hoc analysis evaluating the effect of secukinumab (SEC) on PsA patients with dactylitis, from the FUTURE 5 trial (NCT02404350).4
Methods: FUTURE 5 is a randomized, double-blinded, placebo-controlled, 2-year phase 3 trial in patients with active PsA. Patients (aged ≥18 years) were randomized 2:2:2:3 to s.c. SEC 300 mg or 150 mg with loading dose (LD), 150 mg without loading dose (NL) or placebo. All groups received SEC or placebo at baseline, weeks (wks) 1, 2 and 3 and then every 4 wks from Wk 4. Exploratory efficacy analysis at Wks 16 and 52 included time to resolution of dactylitis by dactylitis severity, proportion of patients with resolution of dactylitis, ACR 20/50 response and Psoriasis Area and Severity Index (PASI) 90.
Results: Of the 996 patients in the trial, 389 (39%) had dactylitis (SEC 150 mg NL [N=103], 150 mg LD [N=80], 300 mg LD [N=82], and placebo [N=124]). The mean (SD) tender joint count, swollen joint count and dactylitis count at baseline was: 24.0 (17.0), 14.5 (11.4) and 4.1 (4.16) and 19.1 (14.8), 9.5 (8.5) and 0, respectively, in patients with (N=389) and without (N=607) dactylitis at baseline. Higher presence of enthesitis, hsCRP and skin disease were also observed in the dactylitis subset. Median time to resolution of dactylitis (days) [95% CI] in patients on SEC 150 mg NL, 150 mg LD, and 300 mg LD, was 85 [61.0, 113.0], 85 [57.0, 119.0] and 57 [30.0, 85.0], respectively. A higher proportion of SEC treated patients achieved resolution of dactylitis, ACR 20/50 and PASI90 responses vs. placebo at Wk16 with further improvements through Wk 52 (Table 1). Resolution of dactylitis based on the number of dactylitis count at baseline (≤2 and ≥3) are shown in Table 2. SEC 300 mg LD was associated with a faster time to resolution of dactylitis and higher ACR50 and PASI90 responses compared to SEC 150 mg.
Conclusion: A higher level of disease burden was observed in patients with dactylitis compared to patients without dactylitis. SEC 300 mg was associated with a faster time to resolution of dactylitis, higher resolution of dactylitis irrespective of severity and higher responses on skin and joints.
References
- Coates, et al. Arthritis Rheumatol. 2016;68:1060–
- Taylor, et al Arthritis Rheum. 2006;54:2665–
- Gladman, et al; J Rheumatol. 2013; 40:1357–59.
- Mease, et al. Ann Rheum Dis. 2018;77:890–97.
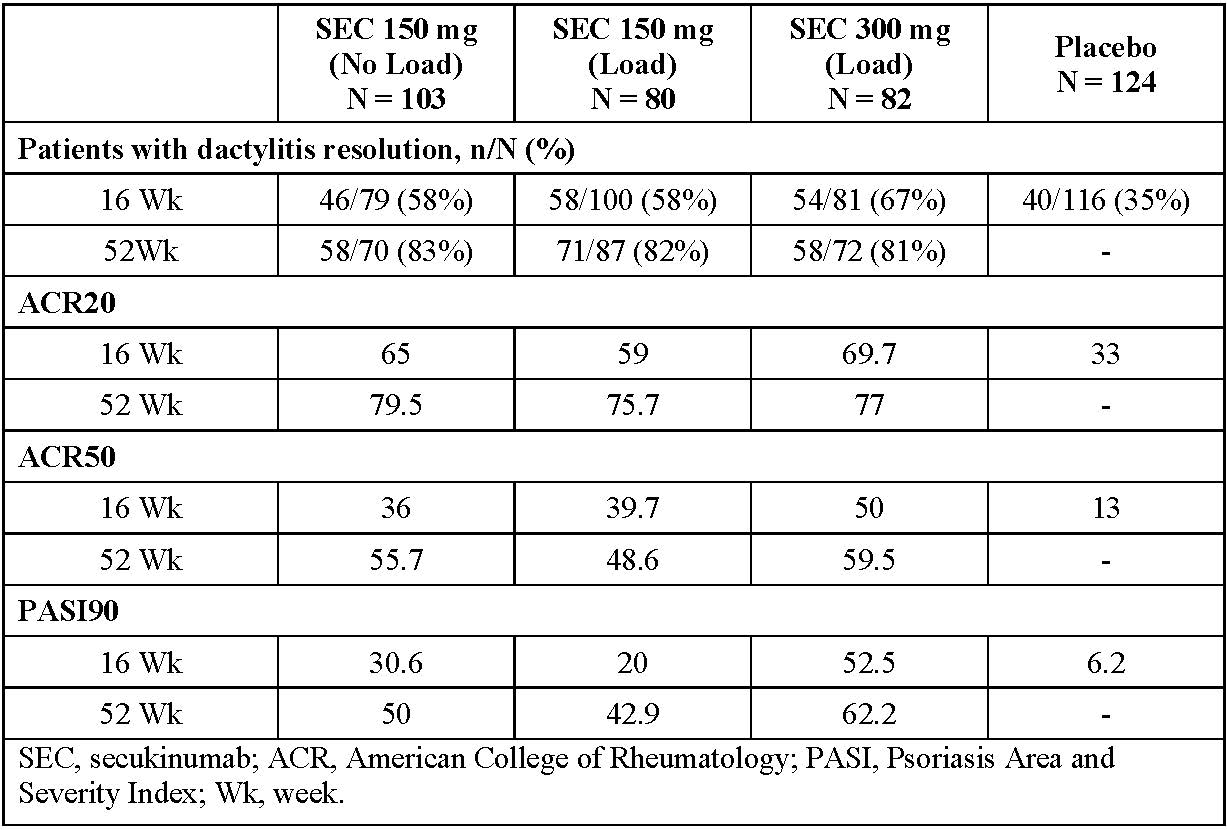 Table 1. Efficacy outcomes in patients with baseline dactylitis (observed data)
Table 1. Efficacy outcomes in patients with baseline dactylitis (observed data)
 Table 2. Efficacy outcomes in patients with dactylitis at baseline ≤ 2 and ≥ 3 (observed data)
Table 2. Efficacy outcomes in patients with dactylitis at baseline ≤ 2 and ≥ 3 (observed data)
To cite this abstract in AMA style:
Kirkham B, Nash P, Navarra S, Quebe-Fehling E, Gaillez C, Sastre C, Mease P. Secukinumab in the Treatment of Dactylitis in Patients with Psoriatic Arthritis: Post Hoc Analysis Results from a Randomized Phase 3 Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/secukinumab-in-the-treatment-of-dactylitis-in-patients-with-psoriatic-arthritis-post-hoc-analysis-results-from-a-randomized-phase-3-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-in-the-treatment-of-dactylitis-in-patients-with-psoriatic-arthritis-post-hoc-analysis-results-from-a-randomized-phase-3-trial/
