Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of psoriatic arthritis (PsA). It was approved in the US in December 2017 for use in combination with non-biologic DMARDs. This analysis of real-world data assessed demographic and baseline clinical characteristics, as well as treatment persistence/adherence, in patients (pts) with PsA who had newly initiated tofacitinib treatment.
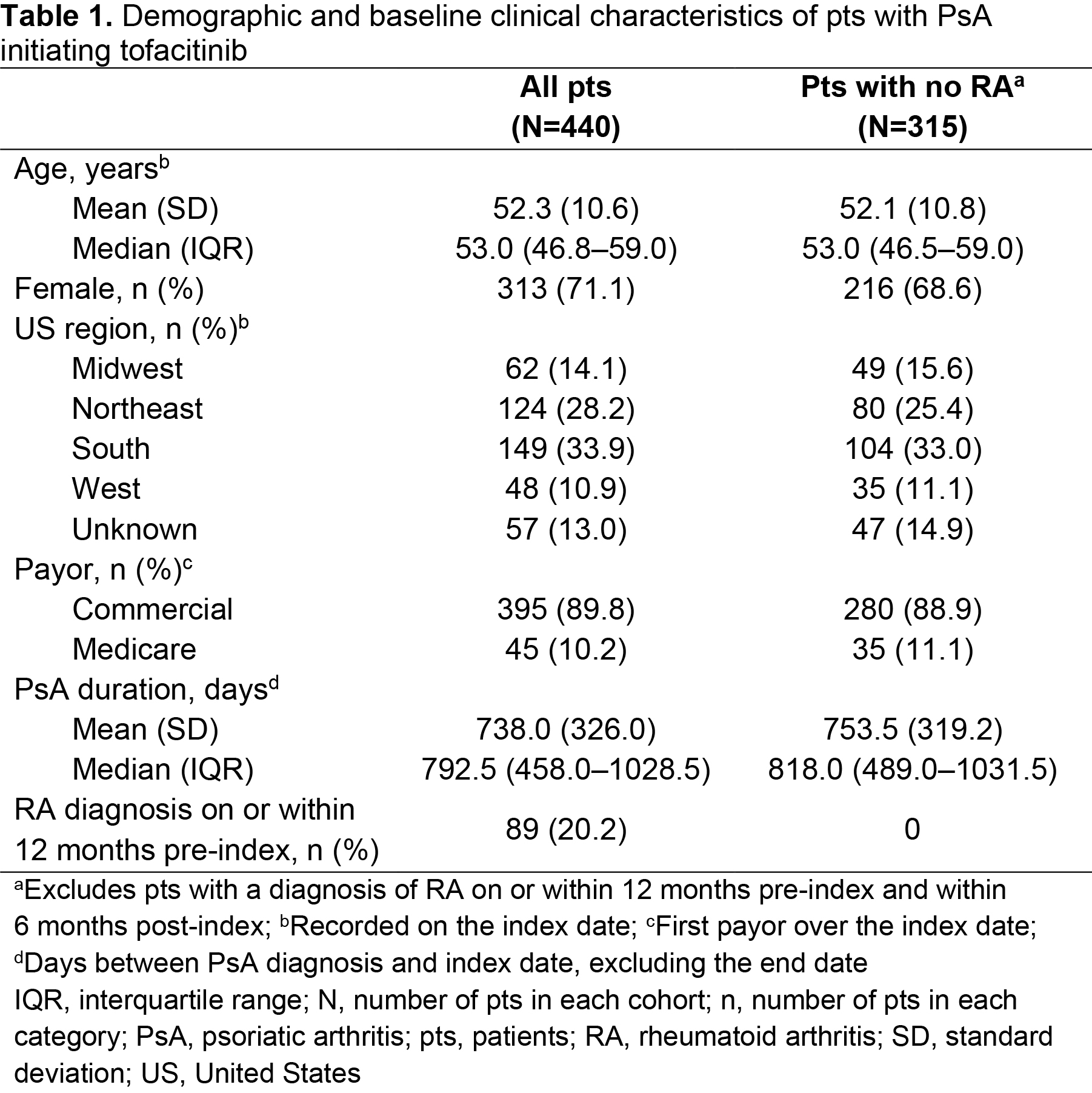
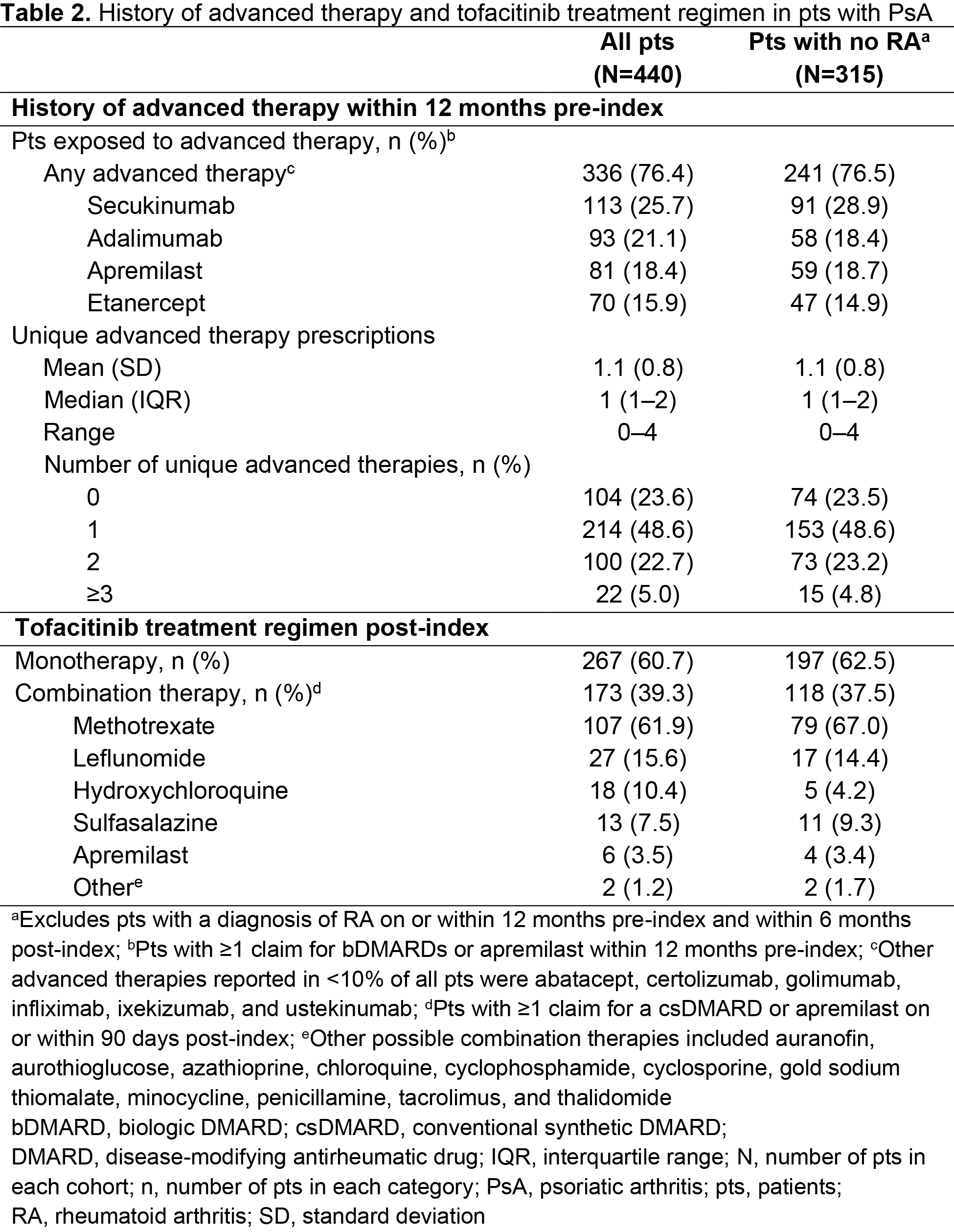
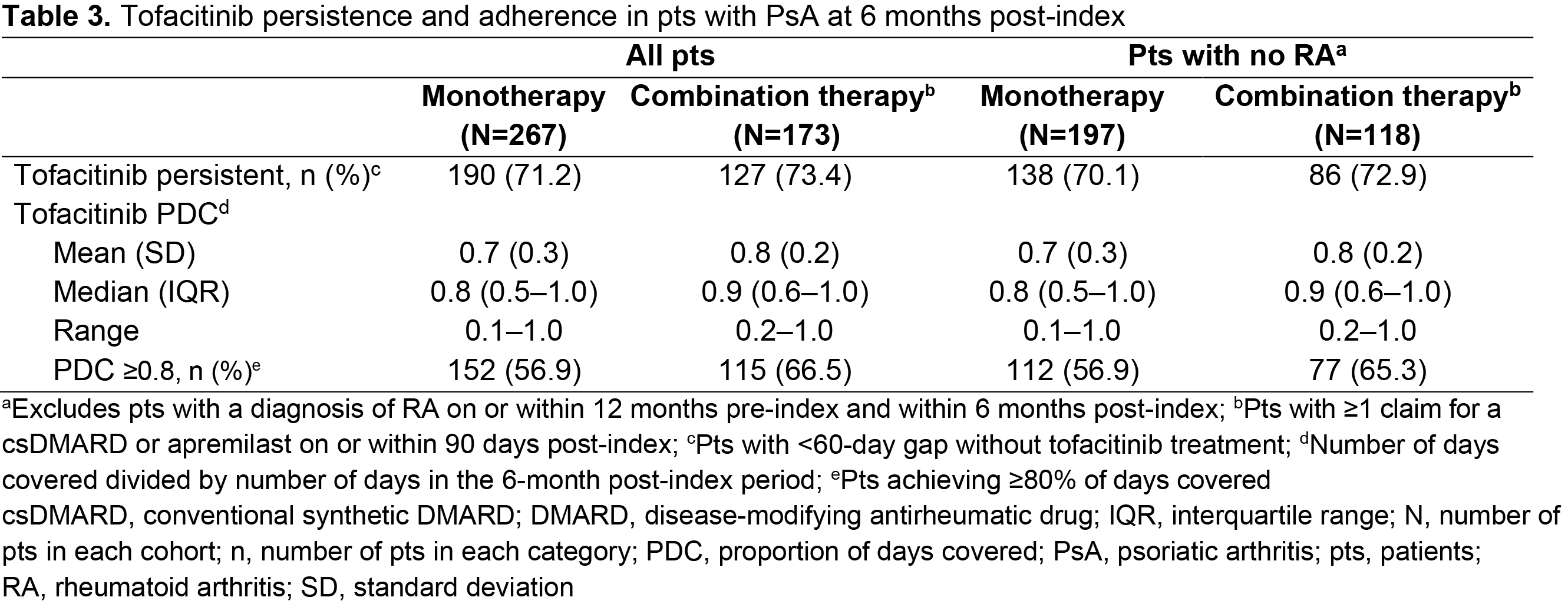
Methods: This retrospective cohort study included pts aged ≥ 18 years in the Truven MarketScan™ US Commercial and Medicare Supplemental Claims and Encounters database with ≥ 1 tofacitinib claim (first = index) between December 14, 2017–April 30, 2019, and PsA diagnoses (≥ 1 inpatient or ≥ 2 outpatient [30–365 days apart]) on or within 12 months pre-index. Pts were continuously enrolled for 12 months pre-index and 6 months post-index, with no pre-index claims for tofacitinib. Pt demographic and clinical characteristics on the day of index, history of treatment with advanced therapies (≥ 1 claim for biologic DMARDs or apremilast within 12 months pre-index), and tofacitinib treatment regimen (monotherapy or combination therapy [≥ 1 claim for conventional synthetic DMARDs or apremilast on or within 90 days post-index]) were recorded. Outcomes at 6 months post-index included tofacitinib persistence (< 60-day gap without tofacitinib treatment) and adherence (proportion of days covered ≥ 80% and medication possession ratio [data not shown]). A sensitivity check was performed by analyzing a sub-cohort which excluded pts with a diagnosis of rheumatoid arthritis (RA) on or within 12 months pre-index and within 6 months post-index.
Results: Of 17,321 pts receiving tofacitinib, 440 pts met the inclusion criteria for the overall cohort, with 315 pts included in the sub-cohort. In the overall cohort, pts were mostly female, with a mean age of 52.3 years and a mean PsA duration of 738 days (Table 1). Most pts were exposed to ≥ 1 advanced therapy (mean = 1.1; range = 0–4) within 12 months pre-index; most commonly secukinumab (Table 2). Overall, 39.3% of patients received tofacitinib combination therapy post-index; most commonly methotrexate (Table 2). Persistence was similar in pts receiving tofacitinib monotherapy (71.2%) vs combination therapy (73.4%; Table 3) at 6 months post-index. Adherence was slightly lower in pts receiving tofacitinib monotherapy (56.9%) vs combination therapy (66.5%; Table 3) at 6 months post-index. All results were similar in the sub-cohort (Tables 1–3).
Conclusion: This analysis of US-based claims data indicated that pts newly initiated tofacitinib treatment an average of 2 years after PsA diagnosis, with the majority ( > 60%) of pts receiving tofacitinib as monotherapy. High levels of persistence and adherence to tofacitinib were observed 6 months after treatment initiation. Findings were similar when pts with PsA who also had a diagnosis of RA were excluded. Data are limited in that claims data cannot confirm that pts took the medication for which they filed a claim for.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Gemma Turner, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Mease P, Young P, Gruben D, Fallon L, Germino R, Kavanaugh A. Early Real-World Experience of Tofacitinib for Psoriatic Arthritis: Data from a United States Healthcare Claims Database [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/early-real-world-experience-of-tofacitinib-for-psoriatic-arthritis-data-from-a-united-states-healthcare-claims-database/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-real-world-experience-of-tofacitinib-for-psoriatic-arthritis-data-from-a-united-states-healthcare-claims-database/