Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical Poster II: Systemic JIA, Autoinflammatory, & Scleroderma
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate the long-term safety profile of anakinra in patients with systemic juvenile idiopathic arthritis (sJIA)
Methods: Data from patients with sJIA according to the ILAR classification criteria, treated with anakinra and enrolled in the Pharmachild registry before 30 September 2018 were analyzed (EUPAS28378). The study endpoints were: the occurrence of non-serious adverse events of at least moderate severity (AEs), serious AEs (SAEs), and events of special interest (ESI) including macrophage activation syndrome (MAS) according to MedDRA version 21.1; the duration of anakinra treatment and the reasons for discontinuation. All endpoints were analyzed overall and stratified by 6 months time windows.
Results: 306 patients were enrolled with both genders equally represented (Table 1). Almost half of the patients (n=146; 46%) were continuously treated with anakinra for at least 12 months, 34.0% for at least 18 months and 28.1% for at least 24 months. A total of 201 AEs was reported during a total of 509.3 patient years (py) of treatment with an overall incidence rate (IR) of 39.5 (95% CI 30.8-50.6) per 100 py , mostly represented by infections (52 events, 25.9%; IR 10.2/100 py). 56 SAEs were reported (IR 11.0/100 py; 95% CI 7.9-15.2), whereof 13 infections (23.2%; IR 2.6/100 py), and 11 MAS episodes (19.6%; IR 2.2/100 py).The proportion of patients experiencing AEs was highest during the first 6 months of treatment and did gradually decrease over time (Table 2). Ten patients (3.3%) had a history of MAS before anakinra start, 9 of these patients did not experience any new MAS episode after anakinra start. 8 patients developed MAS several months after anakinra discontinuation (Table 3). Discontinuation of treatment occurred at least once in 233 patients (76%), more often during the first 6 months, then decreasing over time, and was overall secondary to inefficacy (43%), remission (31%) or AEs and intolerance (15.0%). No deaths occurred during anakinra treatment while 3 deaths occurred after anakinra discontinuation (5 months, 3 years, and 5 years after discontinuation, respectively). No malignancies were reported neither during treatment with anakinra nor after discontinuation .
Conclusion: The results of the present study confirm the long-term safety profile of anakinra in sJIA patients without any new safety findings. Long-term treatment with anakinra in sJIA patients was well tolerated, with a decreasing overall incidence rate of AEs over time.
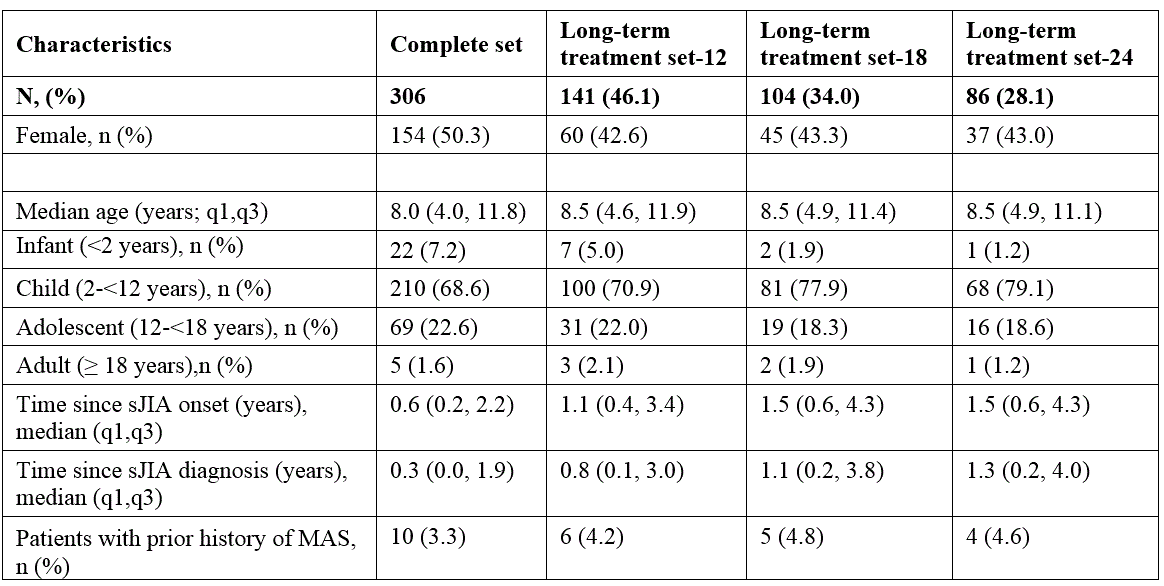 Table 1. Demographics of the study patient population.
Table 1. Demographics of the study patient population.
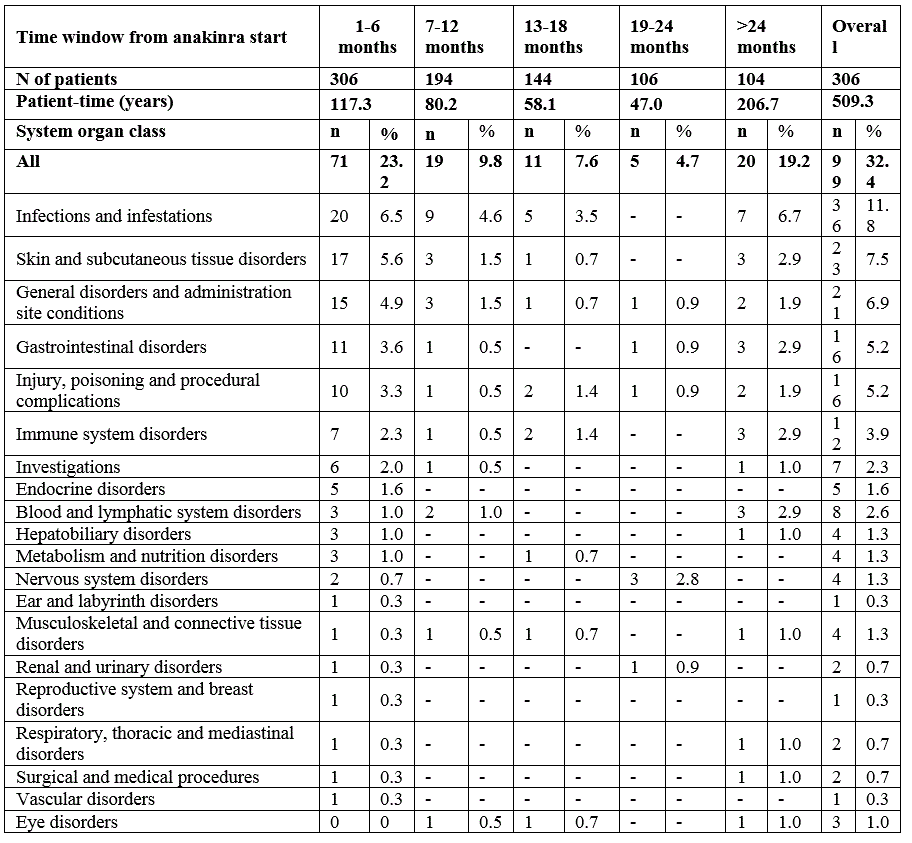 Table 2. Number of AEs and SAEs by time window.
Table 2. Number of AEs and SAEs by time window.
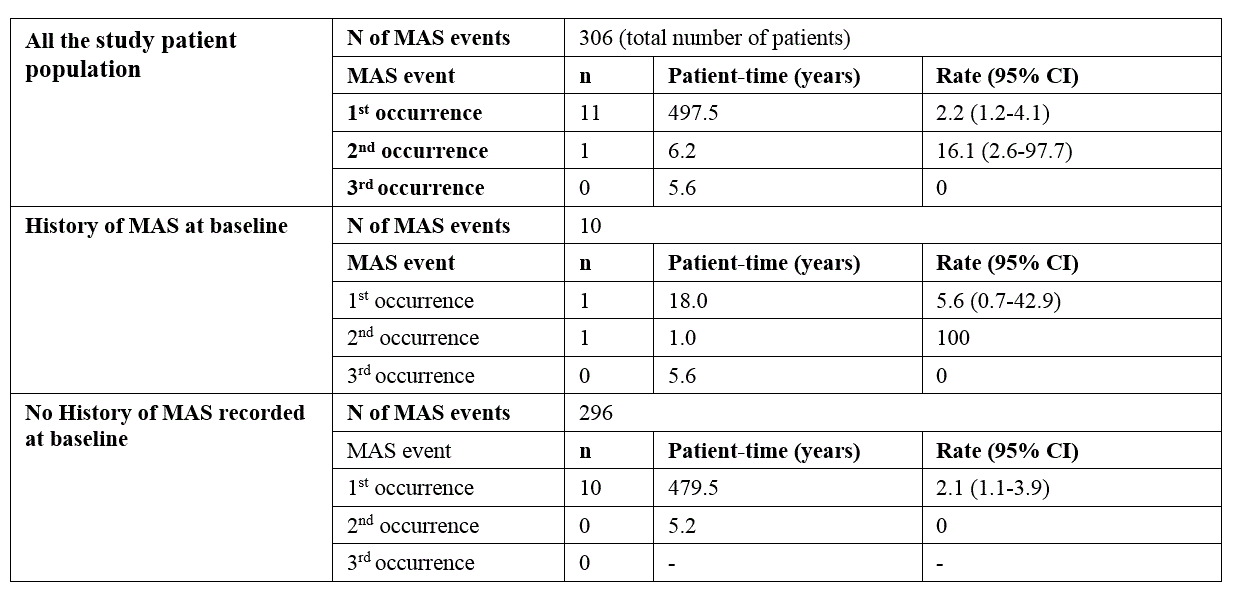 Table 3. Overall MAS events and by history of MAS.
Table 3. Overall MAS events and by history of MAS.
To cite this abstract in AMA style:
Giancane G, Papa R, Vastert S, Bagnasco F, Swart J, Quartier P, Anton J, Kone Paut I, Kamphuis S, Herlin T, Sanner H, De Benedetti F, Tsitsami E, Nielsen S, Moreno E, Pallotti C, Franck-Larsson K, Malmström H, Cederholm S, Wulffraat N, Ruperto N. Long-term Safety Profile of Anakinra in Patients with Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/long-term-safety-profile-of-anakinra-in-patients-with-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-profile-of-anakinra-in-patients-with-systemic-juvenile-idiopathic-arthritis/
