Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment (0985–0989)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Mycophenolate Mofetil (MMF) is standard of care therapy for long term treatment of lupus nephritis and other manifestations of SLE. However, it is associated with adverse effects, pregnancy risks, increased cost, and requirement for routine laboratory monitoring. This randomized, unblinded, study compared the risk of SLE flare in subjects with quiescent disease who withdrew from long-term MMF therapy compared to those continuing maintenance MMF over 60 weeks.
Methods: Adults with quiescent SLE (clinical SLEDAI/cSLEDAI ≤4) on long term MMF therapy (≥ 2 years for nephritis; ≥ 1 year for non-nephritis) were randomized 1:1 to receive unblinded MMF at the baseline dose for 60 weeks (maintenance arm, MA) or to a 12-week structured taper off of MMF (withdrawal arm, WA). All subjects were taking stable doses of hydroxychloroquine; prednisone ≤ 10 mg daily was permitted. Primary endpoint was the difference between arms in the rate of clinically significant disease reactivation (CSDR) by 60 weeks. CSDR was defined as any SELENA-SLEDAI flare and increased immunosuppression (prolonged corticosteroids, resumption or increased dose of MMF, or addition of other immunosuppressives). Rates of BILAG A and B flares, all SLEDAI flares and adverse events were assessed through 60 weeks. Event rates and time to flare were compared using Kaplan-Meier curves.
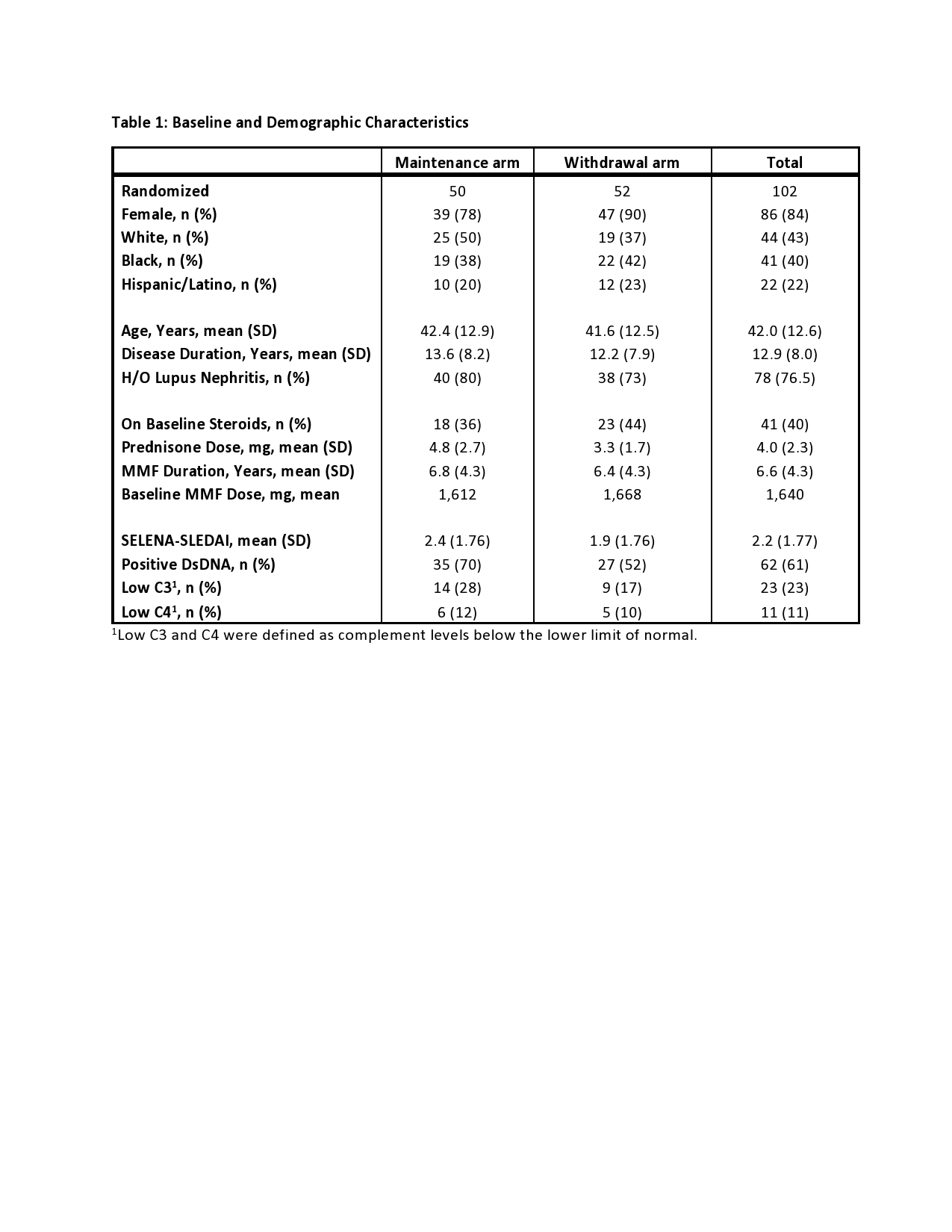
Results: 102 participants were randomized: 50 MA and 52 WA. One subject in each arm was ineligible and 10 subjects terminated early (7 MA, 3 WA). 84% subjects were female, with a mean of 13 years of SLE and 6.6 years of MMF therapy. 76% had a history of lupus nephritis, and mean baseline SLEDAI was 2.2. Five subjects (10%) in the MA experienced a CSDR compared to nine (18%) in the WA with a mean time to CSDR of 38 weeks (median 46 weeks) in both arms. Kaplan Meier estimate of risk difference was 0.07 (95% CI -0.07-0.21). BILAG A disease developed in one subject in the MA (pancreatitis) compared to four in the WA (cranial neuropathy, panniculitis, two renal flares). Kaplan-Meier curves overlapped for CSDR, BILAG A flares, and all SELENA-SLEDAI flares (Figure 1).
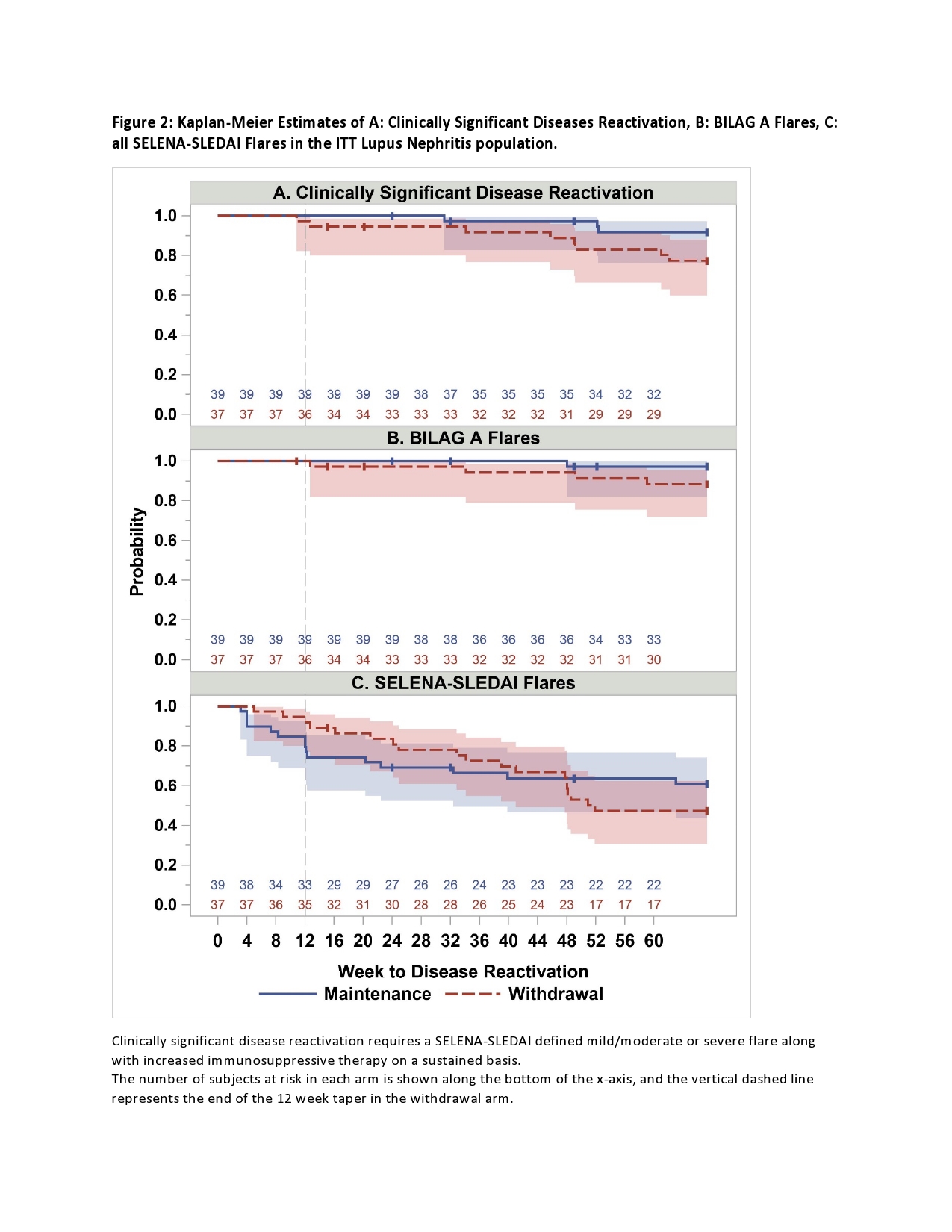
In the participants with history of renal disease (76/100 eligible for analysis), demographics were similar to the entire cohort. Three subjects (7.7%) met CSDR criteria in the MA compared to 8 (22%) in the WA. Kaplan-Meier estimate of risk difference between groups was 0.14 (95% CI -0.02-0.31). BILAG A disease occurred in one in the MA compared to four in the WA. Mean time to CSDR was 45 weeks in the MA compared to 41 weeks in WA. BILAG A disease occurred in one in the MA compared to four in the WA. BILAG A flares and all SELENA-SLEDAI flare Kaplan-Meier curves overlapped between arms. (Figure 2).
Adverse events were similar between groups with Grade 2+ infections occurring more commonly in the MA (63 vs. 49).
Conclusion: In this cohort of subjects with quiescent SLE on long-term MMF therapy, few serious flares occurred in the maintenance or withdrawal groups. Flare rates overlapped between groups, and time to flare was similar. The majority of quiescent SLE patients, including those with a history of lupus nephritis, can safely withdraw long-term MMF therapy.
To cite this abstract in AMA style:
Chakravarty E, Utset T, Kamen D, Contreras G, McCune W, Kalunian K, Aranow C, Clowse M, Massarotti E, Goldmuntz E, Springer J, Keyes-Elstein L, Barry B, Pinckney A, James J. Withdrawal of MMF Is Safe in Quiescent Renal and Non-Renal SLE: Results from a Multi-Center Randomized Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/withdrawal-of-mmf-is-safe-in-quiescent-renal-and-non-renal-sle-results-from-a-multi-center-randomized-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/withdrawal-of-mmf-is-safe-in-quiescent-renal-and-non-renal-sle-results-from-a-multi-center-randomized-trial/