Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor necrosis factor inhibitors (TNFis) are effective treatments for radiographic axial spondyloarthritis (r-axSpA), but may be less effective in patients (pts) without elevated C-reactive protein (CRP). This study evaluated the efficacy of ixekizumab (IXE), a high-affinity monoclonal antibody selectively targeting interleukin-17A, in pts with r-axSpA at 52 weeks (wks) with nonelevated (≤5 mg/L) and elevated ( >5 mg/L) baseline (BL) CRP. Week 16 Assessment of Spondyloarthritis International Society 40% response rate (ASAS40) results comparing IXE to placebo (PBO) stratified by CRP are published.[1]
Methods: COAST-V (NCT02696785) and COAST-W (NCT02696798) were phase 3, multicenter, randomized, double-blind, PBO-controlled trials investigating efficacy of 80-mg IXE every 4 wks and every 2 wks in pts who met ASAS criteria for r‑axSpA, had radiographic sacroiliitis according to mNY criteria, and were biological disease‑modifying antirheumatic drug (bDMARDs)-naïve (COAST-V) or TNFi‑experienced (COAST-W). Data from 157 COAST-V pts and 188 COAST-W pts treated with IXE from Wk 0 to Wk 52 were analyzed. Patients were stratified based on nonelevated (≤5 mg/L) vs elevated ( >5 mg/L) BL CRP. Additional analysis was done with BL CRP ≤10.0 mg/L vs >10.0 mg/L. Efficacy was assessed by ASAS40, ≥50% improvement in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI50), and change in Short Form 36 physical component summary (SF‑36 PCS) score. Missing data were imputed by non‑responder imputation for binary measures and modified BL observation carried forward for continuous measure. Week 16 data are presented for comparison.[1]
Results: Of pts treated with IXE through Wk 52, 34.4% had CRP ≤5.0 mg/L, 65.6% had CRP >5.0 mg/L, 61.8% had CRP ≤10.0 mg/L, and 38.2% had CRP >10.0 mg/L at BL in COAST‑V and 33.0% had CRP ≤5.0 mg/L, 67.0% had CRP >5.0 mg/L, 55.9% had CRP ≤10.0 mg/L, and 44.1% had CRP >10.0 mg/L at BL in COAST-W.
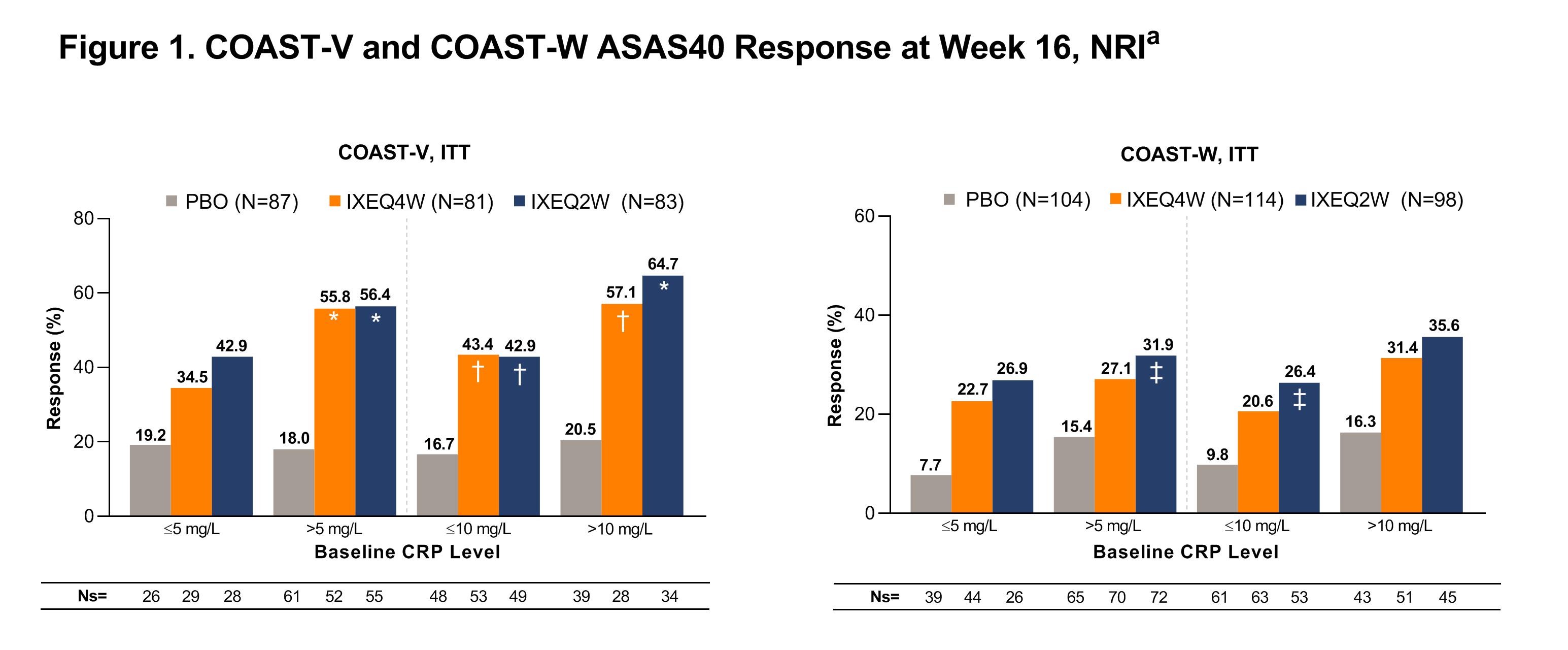
At Wk 16, the proportion of pts achieving ASAS40 in COAST-V was numerically higher with IXE in the ≤5 mg/L group and significantly higher with IXE in the >5 mg/L group vs PBO, as previously shown [1], and was significantly higher with IXE in the ≤10 mg/L and >10 mg/L groups vs PBO (Fig. 1). Results were similar in COAST-W and significant in the >5 mg/L and ≤10 mg/L groups vs PBO (Fig. 1).
At Wk 52, greater than 45% of COAST-V pts and greater than 35% of COAST-W pts treated with IXE achieved an ASAS40 response, greater than 40% of COAST-V pts and greater than 25% of COAST-W pts treated with IXE achieved a BASDAI50 response, and change from baseline in SF-36 PCS score in pts treated with IXE was greater than 5 points in both studies regardless of the BL CRP cutoffs evaluated (Figs. 2 and 3).
Conclusion: A higher proportion of ASAS40 responders was observed in IXE treated arms versus PBO among bDMARD-naïve and TNFi-experienced pts with r-axSpA when the CRP cutoff of 10 mg/L was evaluated, and the responses were consistent through Wk 52. Furthermore, similar proportions of pts achieved BASDAI50 and SF-36 responses within each patient population regardless of the BL CRP cutoff evaluated.
Reference:
1. Maksymowych et al. 2019
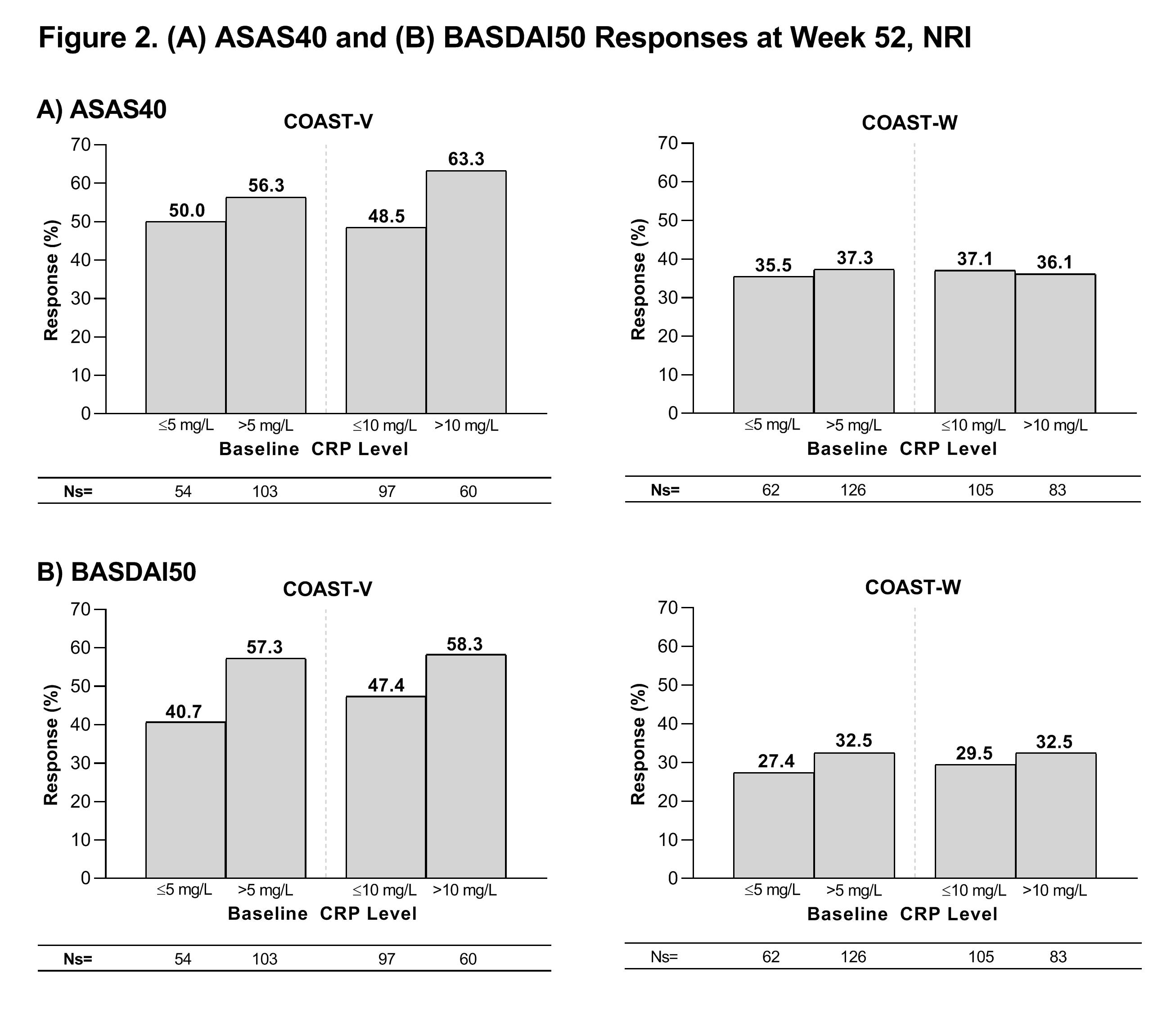 Patients in these analyses received at least one dose of IXE Q4W or Q2W through Week 52. Descriptive statistics are provided without inferential testing. ASAS40=Assessment of Spondyloarthritis International Society 40% response rate; BASDAI50=≥50% improvement in Bath Ankylosing Spondylitis Disease Activity Index; CRP=C-reactive protein; IXE=ixekizumab; NRI=non-responder imputation; Ns=number of patients in each subgroup; Q2W=every 2 weeks; Q4W=every 4 weeks.
Patients in these analyses received at least one dose of IXE Q4W or Q2W through Week 52. Descriptive statistics are provided without inferential testing. ASAS40=Assessment of Spondyloarthritis International Society 40% response rate; BASDAI50=≥50% improvement in Bath Ankylosing Spondylitis Disease Activity Index; CRP=C-reactive protein; IXE=ixekizumab; NRI=non-responder imputation; Ns=number of patients in each subgroup; Q2W=every 2 weeks; Q4W=every 4 weeks.
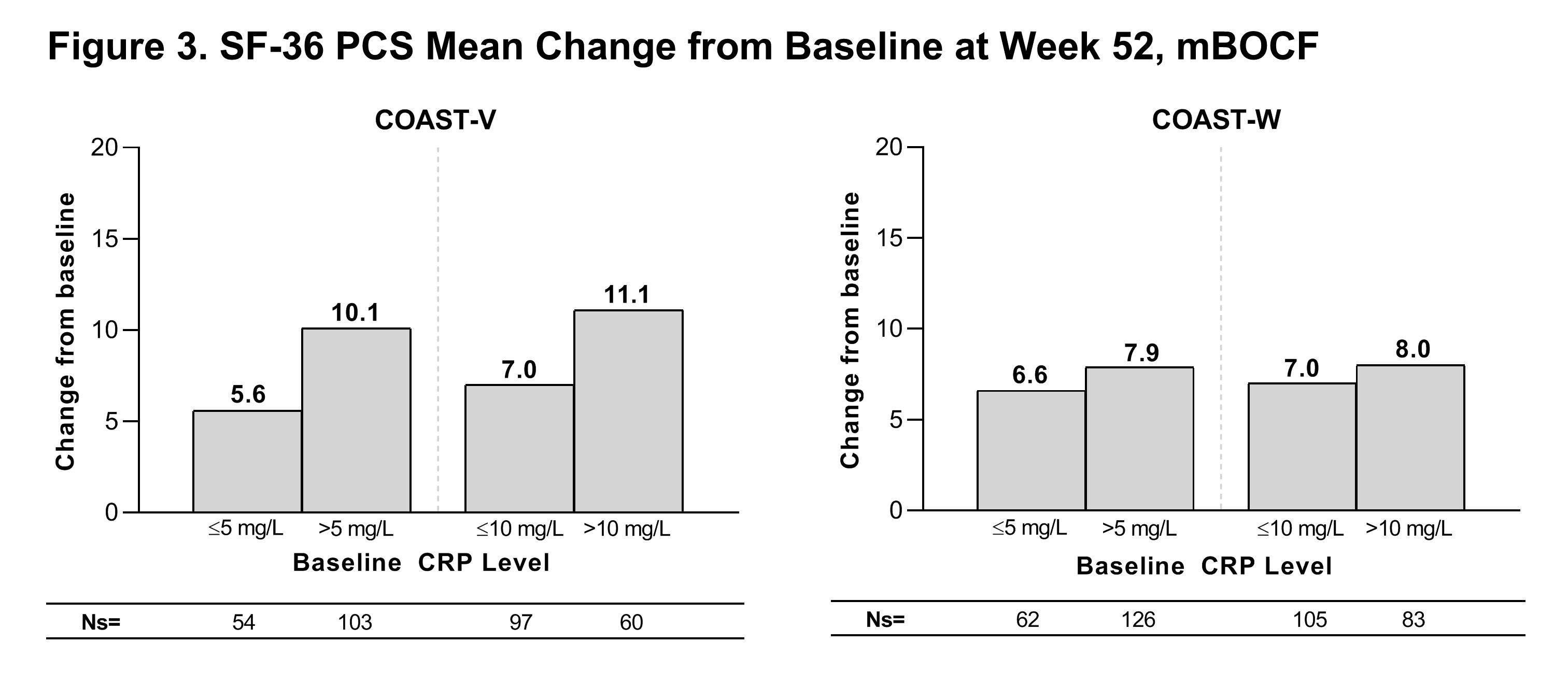 Patients in this analysis received at least one dose of IXE Q4W or Q2W through Week 52. Descriptive statistics are provided without inferential testing. CRP=C reactive protein; IXE=ixekizumab; mBOCF=modified baseline observation carried forward; Ns=number of patients in each subgroup; SF-36 PCS=Short Form 36 Physical Component Score; Q2W=every 2 weeks; Q4W=every 4 weeks.
Patients in this analysis received at least one dose of IXE Q4W or Q2W through Week 52. Descriptive statistics are provided without inferential testing. CRP=C reactive protein; IXE=ixekizumab; mBOCF=modified baseline observation carried forward; Ns=number of patients in each subgroup; SF-36 PCS=Short Form 36 Physical Component Score; Q2W=every 2 weeks; Q4W=every 4 weeks.
To cite this abstract in AMA style:
Reveille J, Rahman P, Sandoval D, Muran T, Kronbergs A, Bolce R, Geneus V, Hunter T, Liu-Leage S, Rudwaleit M, Maldonado-Cocco J, Van den Bosch F. Response to Ixekizumab by C-reactive Protein Level in Patients with Radiographic Axial Spondyloarthritis: Results from the COAST-V (Biological-Naïve) and COAST-W (TNF Inhibitor-Experienced) Trials at 52 Weeks [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/response-to-ixekizumab-by-c-reactive-protein-level-in-patients-with-radiographic-axial-spondyloarthritis-results-from-the-coast-v-biological-naive-and-coast-w-tnf-inhibitor-experienced-trials-at/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/response-to-ixekizumab-by-c-reactive-protein-level-in-patients-with-radiographic-axial-spondyloarthritis-results-from-the-coast-v-biological-naive-and-coast-w-tnf-inhibitor-experienced-trials-at/