Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Ixekizumab, a high-affinity monoclonal antibody that selectively targets interleukin-17A, has demonstrated efficacy in the treatment of the axial spondyloarthritis (axSpA) spectrum (ankylosing spondylitis and non-radiographic axSpA) in patients who are naive to biologic treatments and in those with an inadequate response or intolerance to a tumor necrosis factor (TNF) inhibitor. The objective of this study was to report the long-term safety profile for ixekizumab in patients with axSpA, using integrated safety data from the COAST clinical trial program.
Methods: Safety data from 3 clinical studies and long-term extensions were integrated. Study populations were composed of patients naïve to biological disease-modifying rheumatic disease treatment (bDMARD-naïve) or TNF-experienced, including patients who had intolerance to bDMARDs. The integrated safety population included all patients with axSpA who received ≥1 dose of ixekizumab. Incidence rates (IR) per 100 person-years with 95% confidence interval (CI) and the number of patients were reported. Adverse event (AE) terms were derived from MedDRA (v221.0).
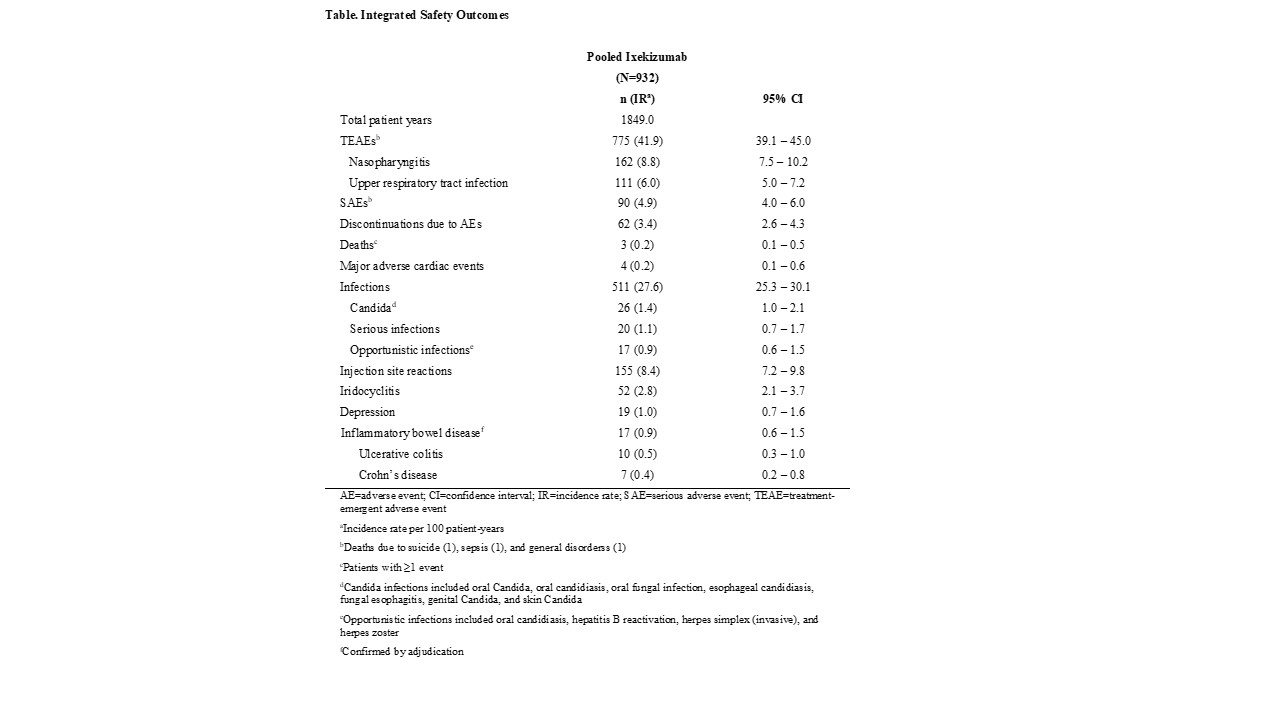
Results: The integrated safety population consisted of 932 patients with a total of 1849 person-years. Among 932 patients with ≥1 TEAE, 775 (IR 41.9, 95% CI 39.1–45.0) TEAEs were reported, of which 95 (IR 5.1, 95% CI 4.2–6.3) were severe (Table). Nasopharyngitis (IR 8.8, 95% CI 7.5–10.2) and upper respiratory infections (IR 6.0, 95% CI 5.0–7.2) were the most commonly reported TEAEs. A total of 90 (IR 4.9, 95% CI 4.0–6.0) SAEs occurred, and 62 AEs led to treatment discontinuation (IR 3.4, 95% CI 2.6–4.3). Injection site reactions occurred in 155 patients (IR 8.4, 95% CI 7.2–9.8). The IR of SAEs declined from year 1 (IR 6.3, 95% CI 4.9–8.3) through 2 years of follow up (IR 4.5, 95% CI 2.8–7.3), as did discontinuations due to AEs (year 1: IR 5.1, 95% CI 3.7–6.8; year ≥2: IR 1.9, 95% CI 0.9–3.9). Allergic reactions/hypersensitivity occurred in 86 patients and decreased slightly from year 1 (IR 6.6, 95% CI 5.1–8.5) through year 3 (IR 3.5, 95% CI 2.0–6.0). The IR of neutropenia (grade 1 or worse) was 8.4 (95% CI 7.2–9.9, and candidiasis (invasive and oral) was 1.4 (95% CI 1.0–2.1). A total of 20 serious infections, 17 reports of adjudicated inflammatory bowel disease, and 1 incidence of potential (unconfirmed) anaphylaxis occurred. No tuberculosis was reported (Table).
Conclusion: This integrated safety analysis demonstrates the overall safety profile of ixekizumab for treatment of axSpA, shows no new signals, and is consistent with that previously reported in previous studies including psoriasis and psoriatic arthritis indications.
To cite this abstract in AMA style:
Schwartzman S, Sandoval D, Kronbergs A, Lisse J, Patel H, Xu W, Liu-Leage S, Magrey M, Marzo-Ortega H, Poddubnyy D. Long-Term Safety Profile of Ixekizumab Treatment in Patients with Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/long-term-safety-profile-of-ixekizumab-treatment-in-patients-with-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-profile-of-ixekizumab-treatment-in-patients-with-axial-spondyloarthritis/