Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) can progress quickly and lead to irreversible damage within 2 years of initial assessment if not treated.1 Secukinumab (SEC), a selective interleukin 17A inhibitor, demonstrated rapid and sustained improvement in the signs and symptoms of PsA in the phase 3 FUTURE 1-5 studies; the mean time since PsA diagnosis (TSD) was 6 to 7 years in these studies.2-6 To better understand the effect of earlier treatment in patients (pts) with PsA, we evaluated SEC treatment in pts with a TSD of ≤ 1 year or > 2 years.
Methods: Data from pts enrolled in FUTURE 2 (NCT01752634), 3 (NCT01989468), 4 (NCT02294227), and 5 (NCT02404350) were pooled and included in this hypothesis-generating analysis (N = 1803). Pts received SEC 300 or 150 mg with subcutaneous loading dose (LD), 150 mg without LD, or placebo (PBO). Pts were classified into 2 groups according to TSD: ≤ 1 year or > 2 years. Response to treatment was assessed using multiple outcome measures, including ACR20/50/70, PASI75/90/100, and additional disease activity and quality of life (QOL) measures at week 16. Responses were calculated using nonresponder imputation. No adjustment was made for multiple comparisons.
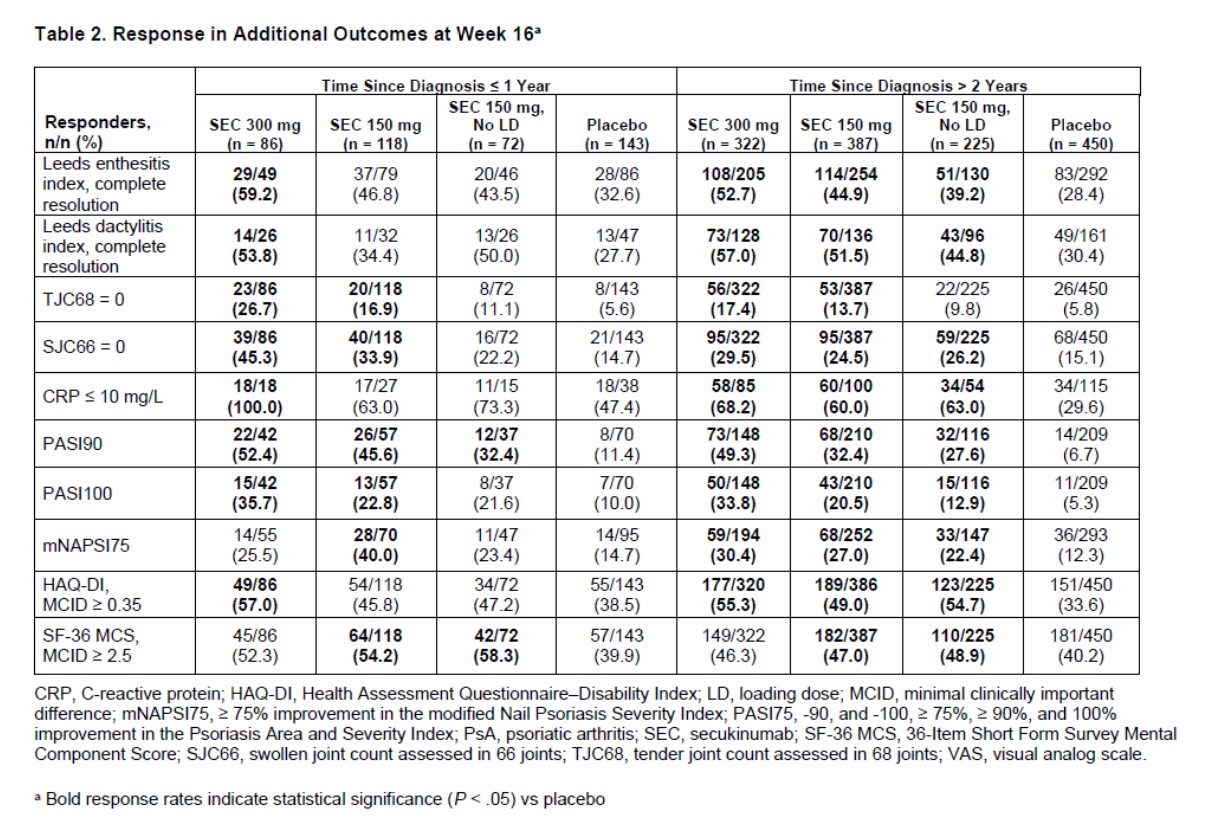
Results: Overall, 419 pts (23.2%) had a TSD ≤ 1 year; 1384 (76.8%) had a TSD > 2 years. At baseline, most pt characteristics were comparable between the 2 TSD groups (Table 1). Dactylitis and prior treatment with TNF inhibitors were more common in pts with a TSD > 2 years at baseline; the mean tender joint count (TJC) was also higher in these pts. At week 16, ACR20/50/70 response rates were higher with SEC than with PBO, regardless of TSD (Figure 1). SEC 300 mg was associated with higher ACR response rates than SEC 150 mg. In general, ACR response rates were slightly numerically higher in pts with a TSD ≤ 1 year, especially in those treated with SEC 300 mg. SEC also led to higher response rates in other efficacy outcomes compared with PBO, including resolution of enthesitis and dactylitis and improvement of skin and nail psoriasis (Table 2). In SEC-treated pts, the proportion of pts with swollen joint count = 0, TJC = 0, CRP ≤ 10 mg/L, and improved SF-36 mental component score were numerically higher in pts with a TSD ≤ 1 year vs those with a TSD > 2 years. Common adverse events in SEC-treated pts (TSD ≤ 1 year/ > 2 years) were nasopharyngitis (8.3%/6.1%), headache (6.2%/3.6%), and upper respiratory tract infection (5.1%/4.7%). Inflammatory bowel disease was reported in 4 pts (SEC, n = 3; PBO, n = 1), all in pts with a TSD > 2 years. Major adverse cardiovascular events occurred in 2 pts (SEC 300 mg, TSD > 2 years). No tuberculosis events were reported.
Conclusion: SEC treatment led to improvement in clinical outcomes and QOL measures in pts with PsA regardless of TSD. Pts with a TSD of ≤ 1 year had higher clinical response rates in some outcome measures, suggesting that earlier treatment may lead to better outcomes in pts with PsA.
References
- Kane D, et al. Rheumatology (Oxford). 2003;42:1460-1468.
- Mease PJ, et al. RMD Open. 2018;4:e000723.
- McInnes IB, et al. Lancet. 2015;386:1137-1146.
- Nash P, et al. Arthritis Res Ther. 2018;20:47.
- Kivitz A, et al. Rheumatol Ther. 2019;6(3):393-407.
- Mease PJ, et al. Ann Rheum Dis. 2018;77:890-897.
To cite this abstract in AMA style:
Ritchlin C, Kivitz A, Nash P, Calheiros R, Meng X, Gaillez C, Kirkham B, McInnes I. Efficacy of Secukinumab Treatment in Patients with Early Psoriatic Arthritis: A Pooled Analysis of 4 Phase 3 Studies [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-secukinumab-treatment-in-patients-with-early-psoriatic-arthritis-a-pooled-analysis-of-4-phase-3-studies/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-secukinumab-treatment-in-patients-with-early-psoriatic-arthritis-a-pooled-analysis-of-4-phase-3-studies/