Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab, a fully human monoclonal antibody that neutralizes interleukin‑17A, has shown long-term efficacy and tolerability in patients with psoriatic arthritis (PsA) in FUTURE 2. Tender joint count (TJC) and swollen joint count (SJC) scores measure synovitis, a key assessment of disease activity. TJC and SJC scores are components of composite measures used to assess joint response to treatment. It is known that response to biologic treatment can be affected by prior and concomitant use of other treatments (e.g. tumor necrosis factor inhibitors [TNFis] or methotrexate [MTX]). Here, we report the 5-year efficacy of secukinumab on reduction of 78 TJC and 76 SJC scores in key FUTURE 2 subgroups: patients naive to or with a prior inadequate response (IR) to TNFis, and patients with and without concomitant MTX use.
Methods: 397 patients with active PsA, who were permitted the use of ≤3 prior TNFis and/or concomitant MTX, were randomized to subcutaneous secukinumab loading dose (300, 150, 75 mg) or placebo at baseline, Weeks 1, 2, 3, and 4, and every 4 weeks thereafter. Patients receiving placebo were re-randomized to secukinumab 300 or 150 mg at Week 16 (non-responders) or 24 (responders). Secukinumab dose could be escalated from 150 to 300 mg or from 75 to 150 or 300 mg starting at Week 128 and maintained thereafter, if active signs of disease were observed based on physician’s assessment. ACR20 response and changes in 78 TJC/76 SJC are reported over 5 years (2 years of core study and 3-year extension) for secukinumab 300 and 150 mg (approved PsA doses) in key subgroups: TNFi-naive, TNFi-IR, and with/without concomitant MTX.
Results: Baseline characteristics were similar across treatment arms (Table 1). At baseline, 33.0%, 37.0%, and 35.7% of patients were TNFi-IR, and 44.0%, 44.0%, and 51.0% were using concomitant MTX in the secukinumab 300, 150 mg, and placebo arms, respectively. The primary endpoint, ACR20 response at Week 24, has been reported previously (McInnes, 2015). In subgroup analyses of the secukinumab 300 and 150 mg arms, 79.6% and 77.1% of TNFi-naive patients achieved an ACR20 response versus 56.3% and 66.7% of TNFi-IR patients at Week 260. At the same timepoint, 62.5% and 73.5% of patients with concomitant MTX use achieved an ACR20 response versus 84.8% and 75.0% of patients without concomitant MTX use in the secukinumab 300 and 150 mg arms, respectively. At Week 260, mean change from baseline in adjusted 78 TJC/76 SJC scores for TNFi-naive and TNFi-IR patients was −13.1/−9.5 and −12.0/−8.9 for secukinumab 300 mg, and −15.3/−8.8 and −14.4/−7.9 for secukinumab 150 mg, respectively (Table 2). At Week 260, mean change from baseline in adjusted 78 TJC/76 SJC scores for patients with and without concomitant MTX was −11.2/−8.9 and −14.4/−9.7 for secukinumab 300 mg, and −14.7/−9.5 and −15.4/−7.5 for secukinumab 150 mg, respectively.
Conclusion: Analysis of 78 TJC and 76 SJC scores demonstrated that treatment with secukinumab provided improvements in synovitis at Week 24, which were sustained over 5 years, irrespective of TNFi history and concomitant MTX use.
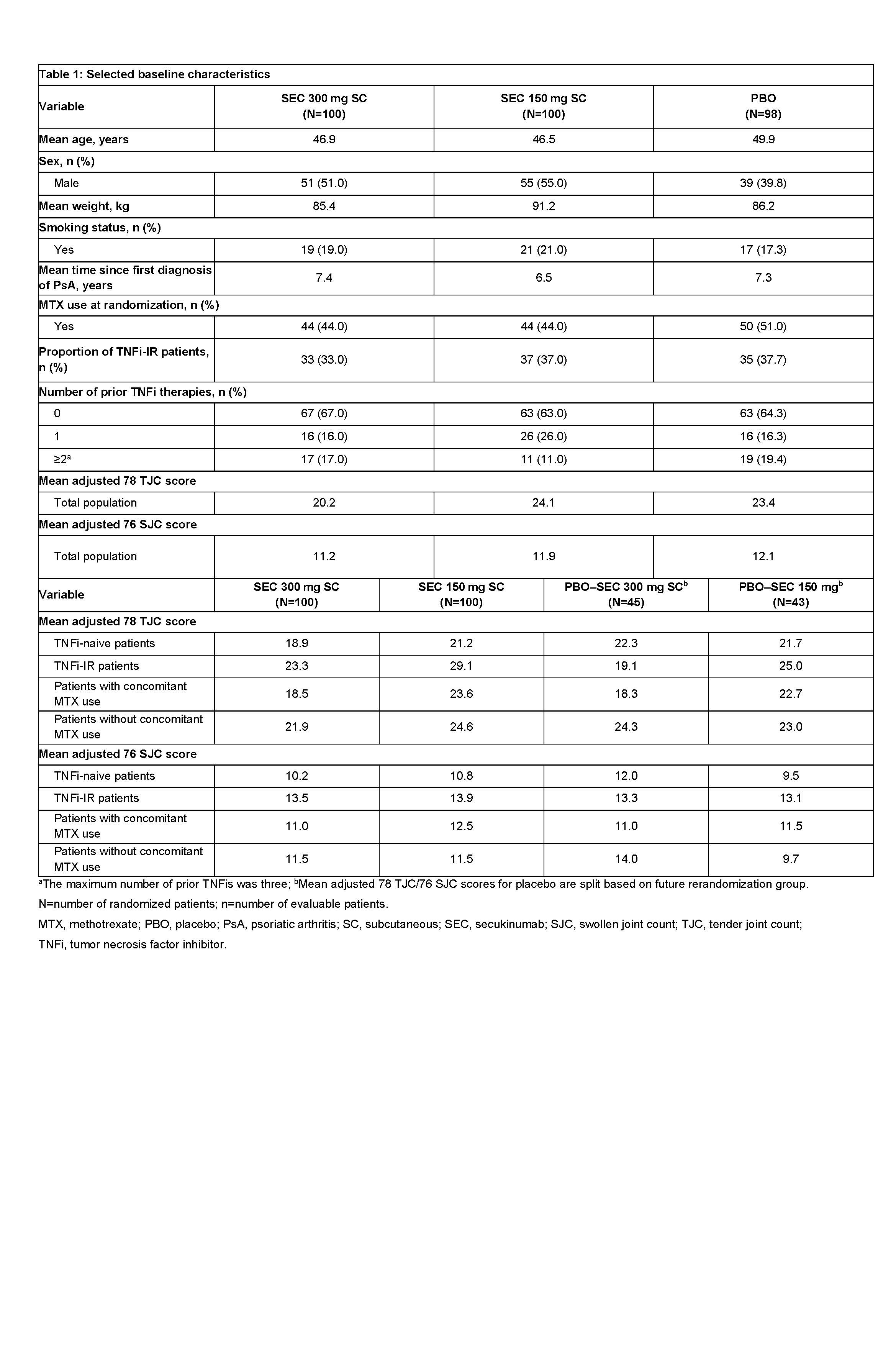 Table 1: Selected baseline characteristics
Table 1: Selected baseline characteristics
 Table 2: 78 TJC and 76 SJC results at Weeks 24 and 260
Table 2: 78 TJC and 76 SJC results at Weeks 24 and 260
To cite this abstract in AMA style:
McInnes I, Chinoy H, Asquith D, White A, Gaillez C. Secukinumab Provides Sustained Improvements in Subgroup Analyses of Joint Tenderness and Swelling in Patients with Psoriatic Arthritis: 5‑Year Results from the Phase 3 FUTURE 2 Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/secukinumab-provides-sustained-improvements-in-subgroup-analyses-of-joint-tenderness-and-swelling-in-patients-with-psoriatic-arthritis-5%e2%80%91year-results-from-the-phase-3-future-2-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-provides-sustained-improvements-in-subgroup-analyses-of-joint-tenderness-and-swelling-in-patients-with-psoriatic-arthritis-5%e2%80%91year-results-from-the-phase-3-future-2-study/
