Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Blockade of IL-6 signaling by sarilumab has been demonstrated to be an effective treatment approach for RA. Due to strict inclusion and exclusion criteria, randomized controlled trials may not represent the heterogeneous RA patient population encountered in regular care. Here we investigated the safety and effectiveness of sarilumab in the treatment of RA in regular care in Germany.
Methods: The prospective, observational, single-arm, 24-month PROSARA study (SARILL08661) is currently under way in Germany at 79 sites, aiming to include up to 750 patients with RA treated with sarilumab. Patients are selected at physician discretion and treated according to label. Study objectives include documentation of safety and various effectiveness outcomes. This interim analysis included patients with data available up to 12 weeks. All analyses are descriptive only.
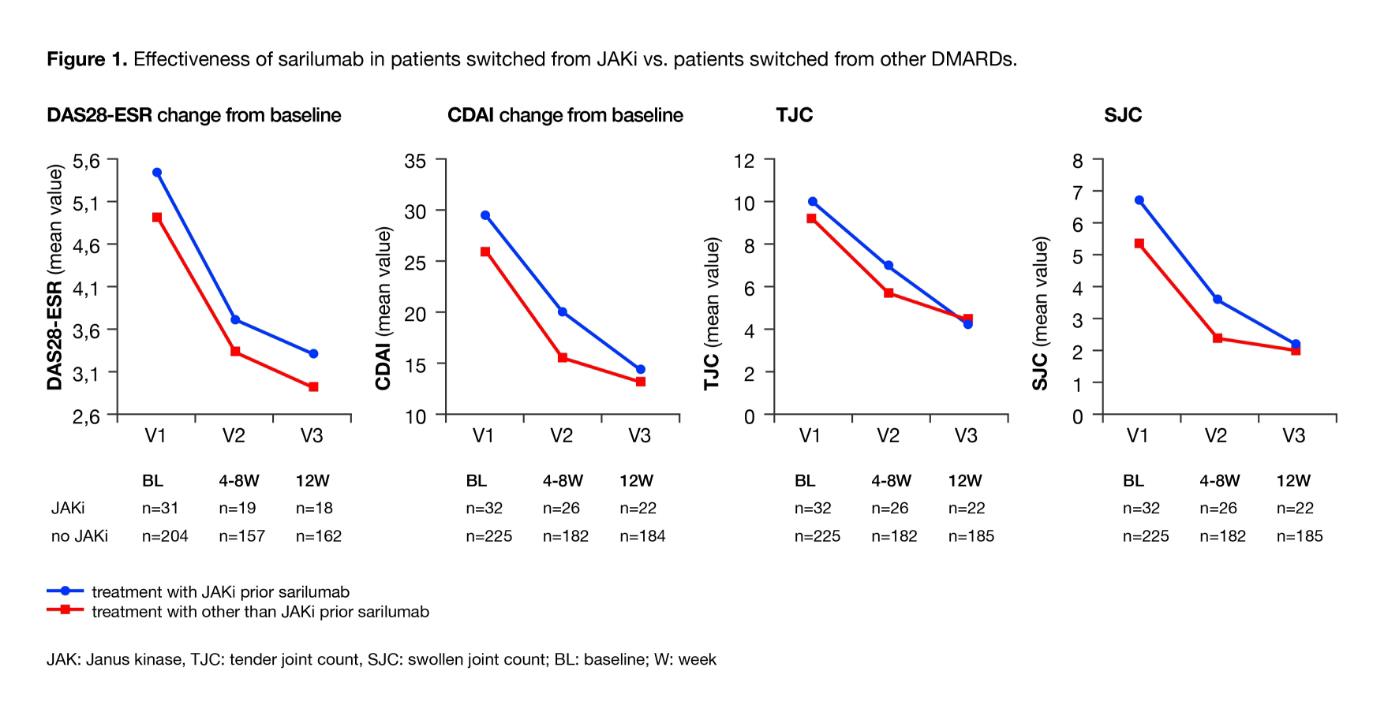
Results: To date 348 patients were included in the study, 304 had baseline data, and 265 also had postbaseline data. The mean age was 59 years (range, 24–83 years); 76% (232/304) were women. Mean disease duration was 10 years; 81% (242/300) of patients had comorbidities. At baseline, 32% (86/265) of patients were biologic- and targeted synthetic DMARD (tsDMARD)-naive. Prior treatments with biologic or tsDMARDs included TNF inhibitors (TNFi; 56% [149/265]), non-TNFi biologics (29% [77/265]), and Janus kinase inhibitors (JAKi, 17% [46/265]). At baseline, 49% (149/304) received sarilumab as monotherapy and 29% (88/304) in combination with conventional DMARDs; combination treatment status was not specified for 22% (67/304) of patients. After 12 weeks of sarilumab treatment, the mean ± SD DAS28-ESR score decreased from 5.0 ± 1.5 to 3.0 ± 1.4 and Clinical Disease Activity Index (CDAI) from 26.7 ± 13.8 to 13.6 ± 11.4. DAS28-ESR remission and low disease activity (LDA) were achieved in 43% (77/180) and 59% (107/180) of patients, respectively; 14% (28/206) and 49% (101/206) reached CDAI remission and LDA. At Week 12, 9% (19/201) of patients had Boolean remission. HAQ-disability index improved from 1.3 at baseline to 1.1 at Week 12 (n=195). Mean CDAI improvement at Week 12 was similar for autoantibody (RF or anticitrullinated protein antibody)-positive and -negative patients (–12.5 vs –15.4, respectively). Patients switching from JAKi to sarilumab (n=32) had longer disease duration than patients who switched from another compound. Of note, similar efficacy was observed between patients who switched from JAKi to sarilumab and those who switched from other DMARDs; disease activity measures (DAS28, CDAI, tender joint count, and swollen joint count) and global assessments improved consistently (Figure 1). Safety was consistent with the anticipated profile of IL-6 receptor inhibition and no new safety signals were observed. Adverse events (AEs) and serious AEs were reported in 34% and 6% of patients, respectively.
Conclusion: Sarilumab administered in regular care was associated with rapid and clinically meaningful improvements in a general RA population, including patients switching from JAKi. The safety profile was consistent with data reported from controlled clinical trials.
To cite this abstract in AMA style:
Feist E, Aries P, Zinke S, Burkhardt H, Albrecht I, Bley O, Obermeier M, Sternad P, Welcker M, Kühne C, Holst A, Baerlecken N, Tony H. PROSARA – A Prospective, Multicenter, Noninterventional Study to Evaluate the Safety and Effectiveness of Sarilumab for the Treatment of Active Rheumatoid Arthritis in Regular Care in Germany [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/prosara-a-prospective-multicenter-noninterventional-study-to-evaluate-the-safety-and-effectiveness-of-sarilumab-for-the-treatment-of-active-rheumatoid-arthritis-in-regular-care-in-germany/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prosara-a-prospective-multicenter-noninterventional-study-to-evaluate-the-safety-and-effectiveness-of-sarilumab-for-the-treatment-of-active-rheumatoid-arthritis-in-regular-care-in-germany/