Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In the 56-week (wk) induction period (IP) of the Phase IIIb Assessing Very Early RA Treatment (AVERT)-2 trial (NCT02504268), a greater proportion of patients (pts) achieved SDAI remission (≤3.3) with SC abatacept (ABA) 125 mg once weekly (QW) + MTX vs ABA placebo (PBO) + MTX.1 Of pts who achieved sustained SDAI remission in the IP and completed a subsequent 48-wk de-escalation (DE) period, ~50% had maintained remission at DE Wk 48.2 However, whether pts were in sustained remission throughout the DE period is not known. This analysis evaluated pt flow during the DE period.
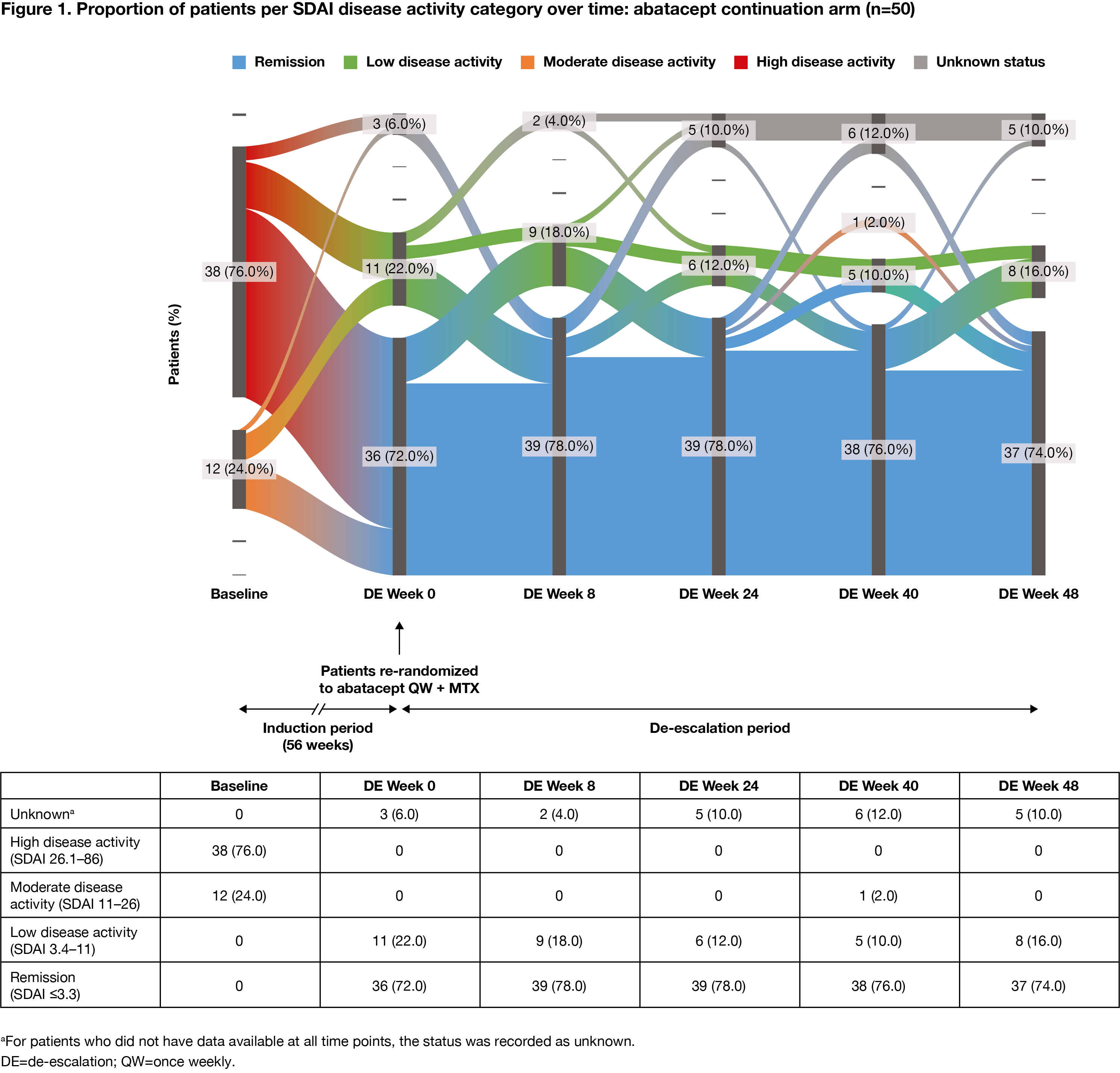
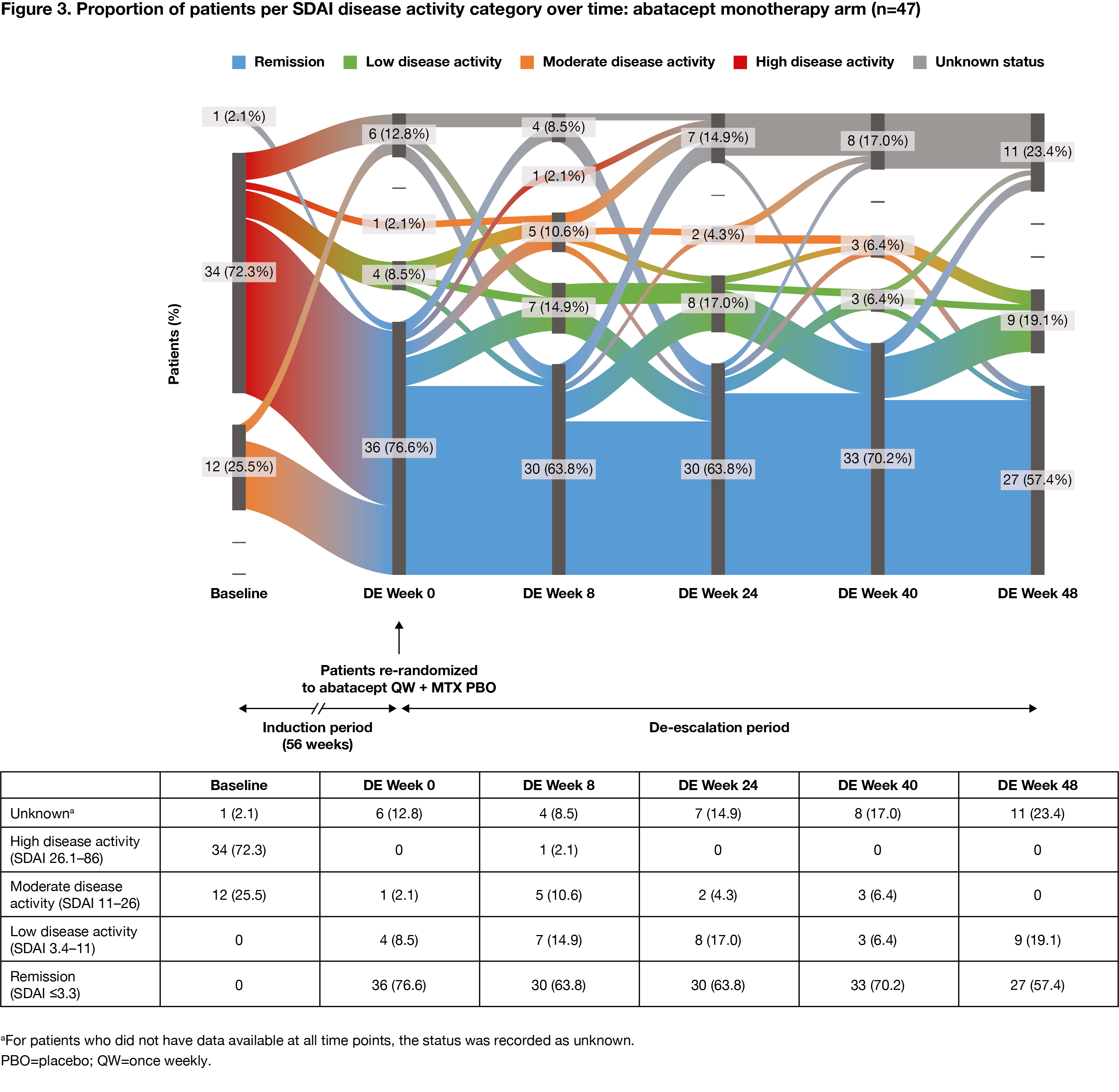
Methods: Pts who met the inclusion criteria (age ≥18 years [yrs]; RA diagnosis [ACR/EULAR 2010 criteria]; RA duration ≤6 months; SDAI >11; ACPA+; elevated CRP >3 mg/L or ESR ≥28 mm/h; TJC ≥3 and SJC ≥3; DMARD naïve) were enrolled in the 56-wk IP of AVERT-2 and received 1 yr of blinded treatment with ABA QW + MTX or ABA PBO + MTX. Pts who completed induction with ABA + MTX and had sustained SDAI remission (≤3.3 at both Wks 40 and 52 of the 56-wk IP) entered the DE phase and were re-randomized 1:1:1 to ABA QW + MTX for 48 wks (ABA continuation), ABA every other wk (EOW) + MTX for 24 wks then ABA PBO + MTX for 24 wks (DE/withdrawal), or ABA QW + MTX PBO for 48 wks (ABA monotherapy). Pts in sustained SDAI remission on ABA PBO + MTX during the IP were not re-randomized and continued the same blinded treatment in the DE phase (MTX monotherapy). MTX and oral CS doses were maintained in the DE period. Proportion of pts in each SDAI disease activity category (remission, low, moderate, high) was assessed over time (intent-to-treat analysis). Flow of disease activity to DE Wk 48, among re-randomized patients, was plotted using Sankey diagrams.
Results: The proportion of pts with high disease activity at IP baseline, by DE treatment arm, ranged from 72% to 76% for ABA + MTX-treated pts (DE re-randomization). Among pts who had achieved sustained SDAI remission in the IP, at the start of the DE period, 147 ABA + MTX-treated pts were re-randomized to ABA continuation (n=50), DE/withdrawal (n=50) or ABA monotherapy (n=47), and 37 pts assigned MTX monotherapy during the IP continued treatment in the DE period. During the DE period, fewer changes in disease activity were seen with ABA continuation versus DE/withdrawal or ABA monotherapy. The proportions of patients in SDAI remission were: ABA continuation: 72% at Wk 0, 74% at Wk 48 (Figure 1); ABA DE/withdrawal: 88% at Wk 0, 74% at Wk 24 for ABA EOW + MTX and 48% at Wk 48 after ABA withdrawal (Figure 2); ABA monotherapy: 77% at Wk 0, 57% at Wk 48 (Figure 3).
Conclusion: Fewer changes in disease activity were observed when IP treatment was maintained during the DE period. Most pts with high disease activity at IP baseline who achieved SDAI remission sustained remission during the DE period, suggesting that DE is possible once sustained remission has been achieved. Continued treatment with abatacept QW + MTX provided the best outcome.
References
- Emery P, et al. Arthritis Rheumatol 2018;70(suppl 10):602–604.
- Emery P, et al. Arthritis Rheumatol 2019;71(suppl 10):5241–5242.
Medical writing: Lola Parfitt (Caudex)
To cite this abstract in AMA style:
Emery P, Tanaka Y, Bykerk V, Bingham III C, Huizinga T, Citera G, Huang K, Connolly S, Elbez Y, Wong R, Lozenski K, Fleischmann R. Clinical Responses and Patient Flow over 2 Years of Treatment with Abatacept, Including Dose De-Escalation, in Patients with Early, MTX-Naïve, ACPA+ RA: Results from a Phase IIIb Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/clinical-responses-and-patient-flow-over-2-years-of-treatment-with-abatacept-including-dose-de-escalation-in-patients-with-early-mtx-naive-acpa-ra-results-from-a-phase-iiib-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-responses-and-patient-flow-over-2-years-of-treatment-with-abatacept-including-dose-de-escalation-in-patients-with-early-mtx-naive-acpa-ra-results-from-a-phase-iiib-study/