Session Information
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: Coronavirus disease (COVID-19) infected patients present with a state of ongoing inflammation and an exaggerated inflammatory state due to unregulated cytokine release called the cytokine storm syndrome (CSS).Early identification and timely, management of CSS is needed for avoiding an untoward outcome. Multi-disciplinary involvement is needed because of the complexity of the syndrome and the multi-systemic presentation. We summarize a novel approach and our experience in the formation and functioning of multidisciplinary COVID-19 CSS task force at Montefiore Medical Center in the Bronx, New York City (NYC).
Methods: As adult and pediatric rheumatologists, allergy and immunologists ( AI) are well versed with managing inflammatory disorders, after input of infectious disease (ID) and critical care team (CCM) specialists, with consensus developed a set of 4 clinical and 6 laboratory parameters to potentially help in early identification of COVID 19 patients progressing towards CSS
Every consulted patient was reviewed by a sub-specialist and discussed in daily multidisciplinary virtual conference. A consensus recommendation was made regarding steroid therapy or biologic therapy if deemed appropriate. All biological therapy recommended by the CSS task force required a final approval from designated physician from ID or CCM.
Results: Between April 4,2020 and May 7,2020, the CSS consult service evaluated a total of 288 patients. Patients who received biologic therapy were younger with a median age of 53 years (IQR= 32-63 years) versus 62.5 years (52-70 years) in the no-biologic group (p=0.008). A higher proportion of patients receiving biologics were in the critical care setting {26 (84%) vs 151 (59%), p= 0.006}, were on mechanical ventilation {26 (84%) vs 146 (57%), p=0.003} and on vasopressors {22 (71%) vs 111 (43%), p=0.003}. Patients who received biologics were more likely to have a longer median duration of steroid therapy 12.5 days (IQR= 6-15 days) vs 8 days (IQR= 5-12 days) (p=0.01).
Conclusion: To our knowledge, this is the first multi-disciplinary team model collaborative effort to provide consensus recommendations for COVID-19 patients at risk of developing CSS, which is a unique experience at our institution where multiple sub-specialities came together to create a consult service to help early identify and treat patients with COVID-19 CSS, at a time of unparalleled COVID-19 surge with limited resources in NYC.
 Table 1: Clinical and Laboratory Parameters based on consensus recommendations of the COVID-19 CSS Task Force.
Table 1: Clinical and Laboratory Parameters based on consensus recommendations of the COVID-19 CSS Task Force.
 Table 2: Characteristics of COVID-19 patients evaluated by the CSS Task Force
Table 2: Characteristics of COVID-19 patients evaluated by the CSS Task Force
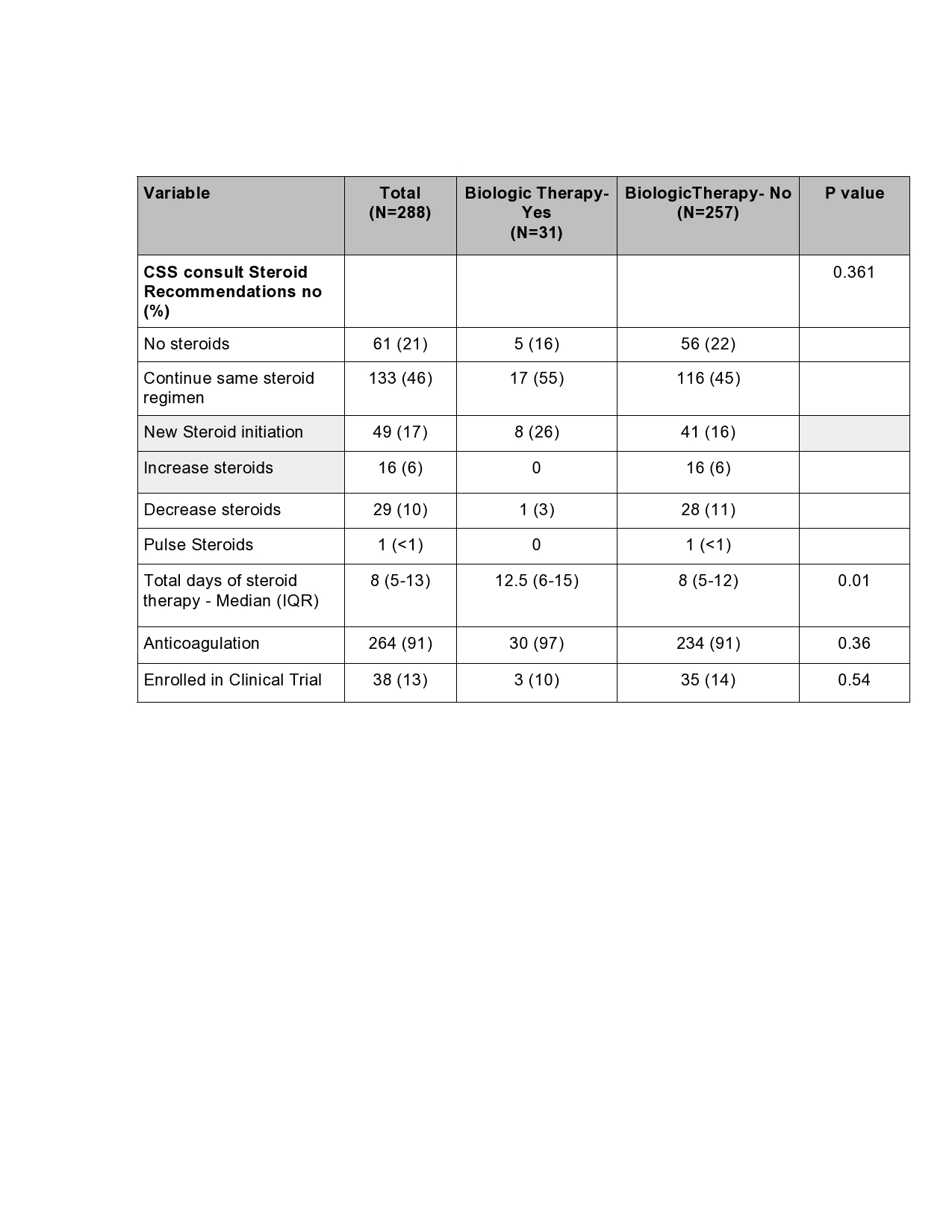 Table 3: Treatment Profile of Patients Evaluat-ed by the COVID-19 CSS Task Force
Table 3: Treatment Profile of Patients Evaluat-ed by the COVID-19 CSS Task Force
To cite this abstract in AMA style:
Ayesha B, Kumthekar A, Jain R, Patel S, Ramesh M, Ferastraoaru D, Hudes G, Karagic M, Zafar S, Bartash R, Vasquez-Canizares N, Kitsis E, Tagoe C, Wahezi D, Rubinstein T, Broder A. Rapid Implementation of a Multidisciplinary COVID-19 Cytokine Storm Syndrome Task Force [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/rapid-implementation-of-a-multidisciplinary-covid-19-cytokine-storm-syndrome-task-force/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rapid-implementation-of-a-multidisciplinary-covid-19-cytokine-storm-syndrome-task-force/
