Session Information
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Baricitinib has been used to treat pediatric patients (pts) with Type 1 Interferonopathies1. Safety profile including BK viral reactivation in urothelium and pharmacokinetic model have been reported1,2. Given the association of BK nephropathy with BK viremia3, we assessed long term safety and consequences of chronic BK viruria on renal function.
Methods: Between October 2011 and August 2018, 25 pts [10 CANDLE (Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures), 7 SAVI (Stimulator of IFN genes–associated [STING-associated] vasculopathy with onset in infancy) and 8 other interferonopathies] were enrolled in an institutional review board approved protocol (NCT01724580). Safety was longitudinally assessed during study period (cut off May 2020). After detection in an index pt in June 2015, BK viral load and renal function were monitored longitudinally, with baseline assessments in 7 additionally enrolled pts. Dichotomous adverse events (AEs and SAEs) were descriptively analyzed, for continuous outcomes (BK viral load) paired t-tests were used.
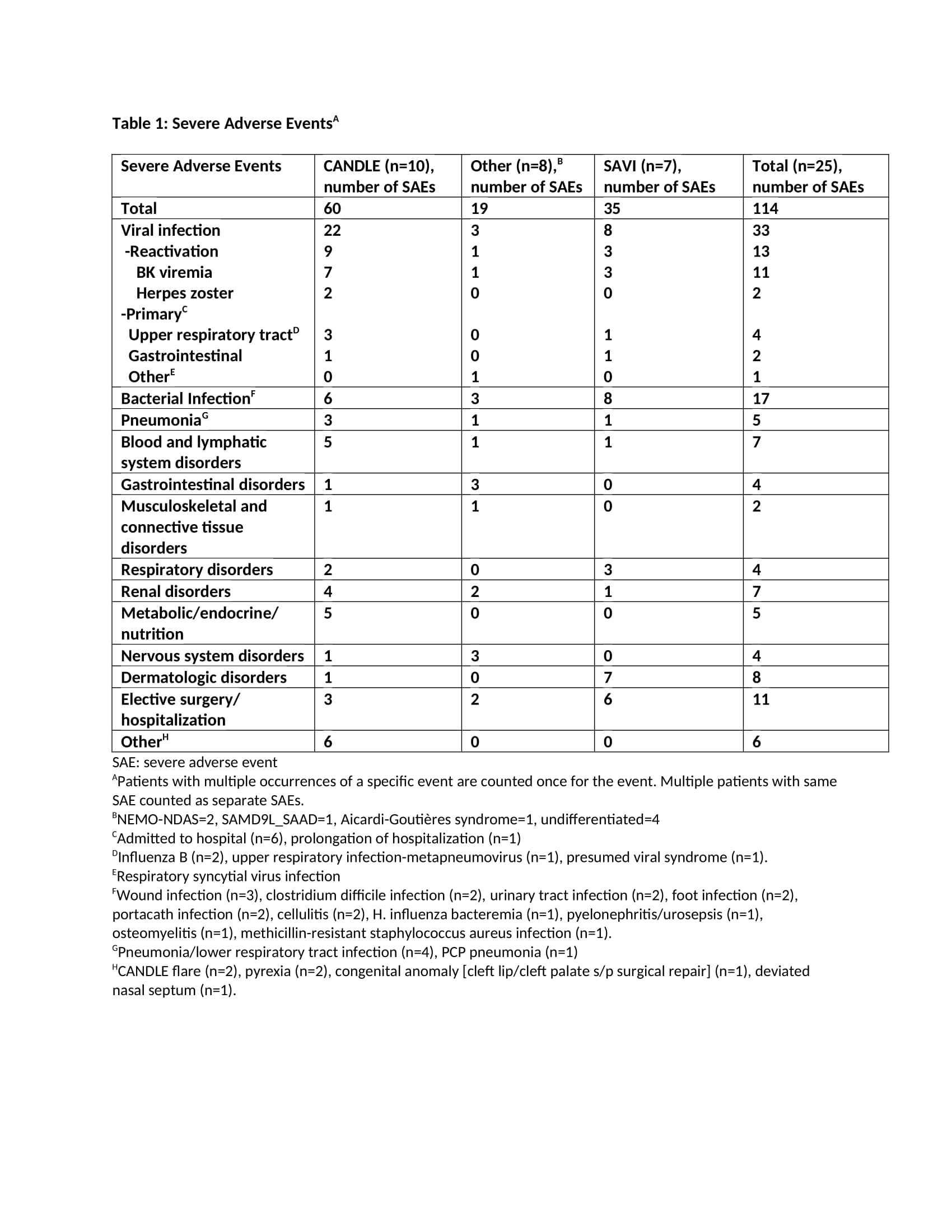
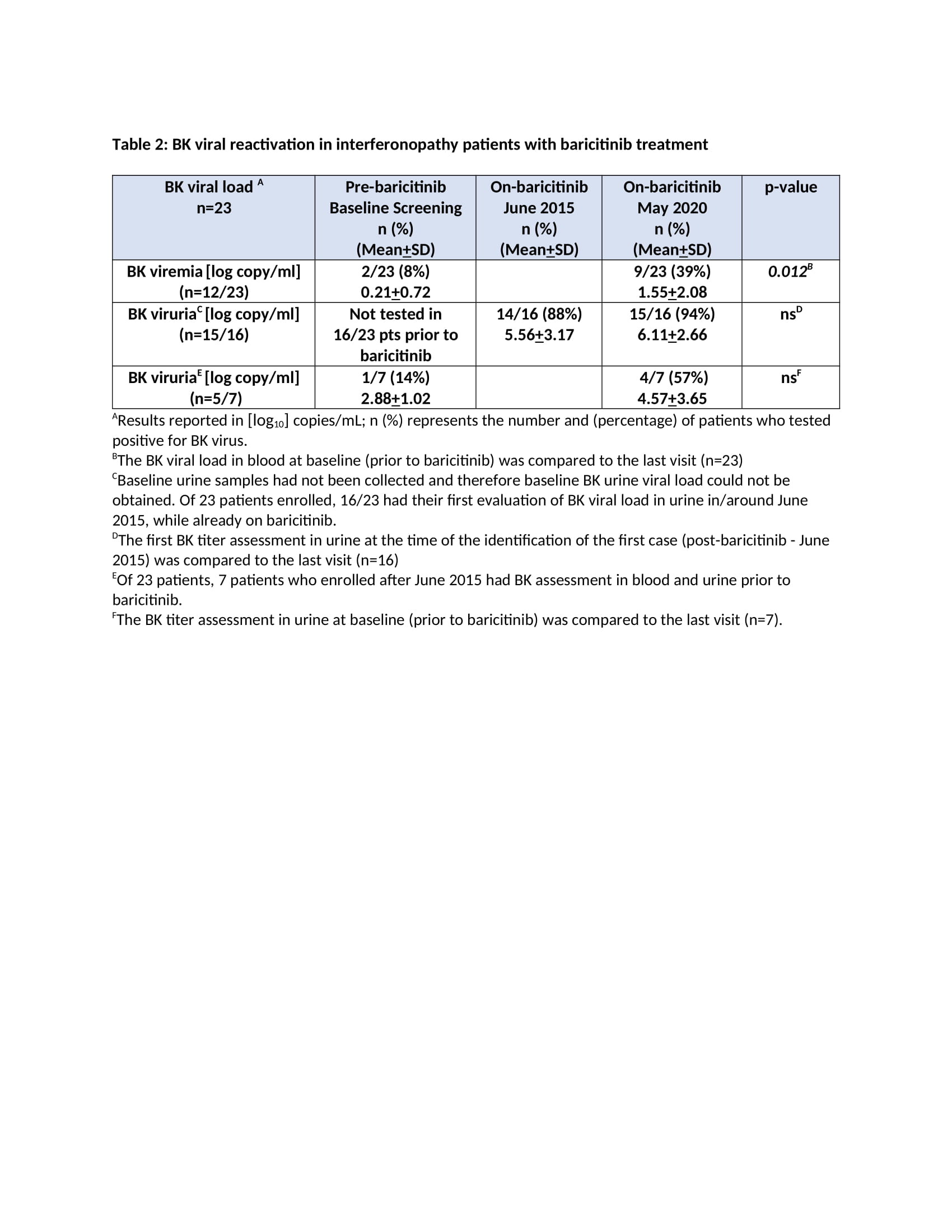
Results: The mean age at enrollment was 11.5 years [range 1.2-24.2]. The mean baricitinib exposure was 4.2 years [104.6 pt.-years]. Of 25 pts, 1 CANDLE (azotemia in the context of BK viremia) and 4 pts with undifferentiated disease discontinued (4/4 inadequate response; 1/4 osteonecrosis). Nine pts are receiving commercial baricitinib and discontinued from study. During the study period, 114 SAEs were reported [66 were treatment related] (Table 1). Of 23 pts monitored, 20 developed BK viruria (1/20 pre-baricitinib). All except the index pt. had stable renal function over a mean of 4.9+2.4 years on baricitinib. Out of 8 pts with no BK viruria at baseline, 5 (63%) developed viruria after a mean of 8.3+10.3 months on baricitinib at a mean age of 5.8+2.3 years. Two pts had BK viremia prior to baricitinib (1 on tacrolimus and 1 azathioprine) and 10 additional pts developed viremia [mean 3.08+1.1 log copy/ml] after a mean of 1.7+1.4 years on baricitinib. The mean urine BK load was 7.7+2 log copy/ml at the time of first viremia (Table 2). Herpes zoster developed in 2 pts and resolved without holding baricitinib. The most frequent treatment-emergent infectious AEs were upper respiratory tract infections, gastroenteritis, sinusitis and pneumonia (in 76%, 36%, 36%, 28% of the pts respectively).
Conclusion: Overall, baricitinib was well tolerated. BK reactivation in urine occurred or was present in 20/23 (87%) pts. In 19/23 who were prospectively monitored, baricitinib dose adjustments to keep BK viremia negative or low were made and renal function remained unchanged during the study period. Our data suggest that BK viral load should be monitored on baricitinib. The presence of BK viremia/ viruria prior to baricitinib treatment suggests evaluation of BK viral load in pts on other chronic immunosuppressive regimens.
Acknowledgements:
This work was supported by Eli Lilly under a CTA and funding for this study was provided by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Ref:
1. Montealegre Sanchez GA et al. J Clin Invest 201
2. Kim H et al. Clin Pharmacol Ther 2018
3. Hamasaki Y et al. Pediatr Transplant 2019
To cite this abstract in AMA style:
Cetin Gedik K, Souto Adeva G, Wade J, Montealegre Sanchez G, de Jesus A, Goldbach-Mansky R. Monitoring of BK Reactivation and Long-term Safety on JAK1/2 Inhibition with Baricitinib [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/monitoring-of-bk-reactivation-and-long-term-safety-on-jak1-2-inhibition-with-baricitinib/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/monitoring-of-bk-reactivation-and-long-term-safety-on-jak1-2-inhibition-with-baricitinib/