Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Apremilast (APR) was associated with improvements in physician-assessed and patient-reported outcomes in a large cohort of patients with PsA based on a 1-year interim analysis of LAPIS-PsA, a real-world study conducted in clinical practice settings in Germany. The current interim analysis of LAPIS-PsA evaluated the effectiveness and safety of APR in patients with and without prior biologic therapy.
Methods: LAPIS-PsA is a multicenter, prospective, non-interventional study enrolling patients with PsA under routine care in Germany who initiated APR according to the summary of product characteristics. Assessments included the Physician’s Global Assessment of Disease Activity (PhGA), Patient’s Global Assessment of Disease Activity (PtGA), Leeds Enthesitis Index (LEI), dactylitis count, swollen and tender joint counts (SJC/TJC), and Psoriatic Arthritis Impact of Disease (PsAID). Efficacy was examined descriptively at baseline and Visit 1 (~1 month), Visit 2 (~4 months), Visit 3 (~7 months), Visit 4 (~10 months) and Visit 5 (~52 weeks). Safety was evaluated based on adverse events (AEs) in the safety population (all patients who received ≥1 dose of APR).
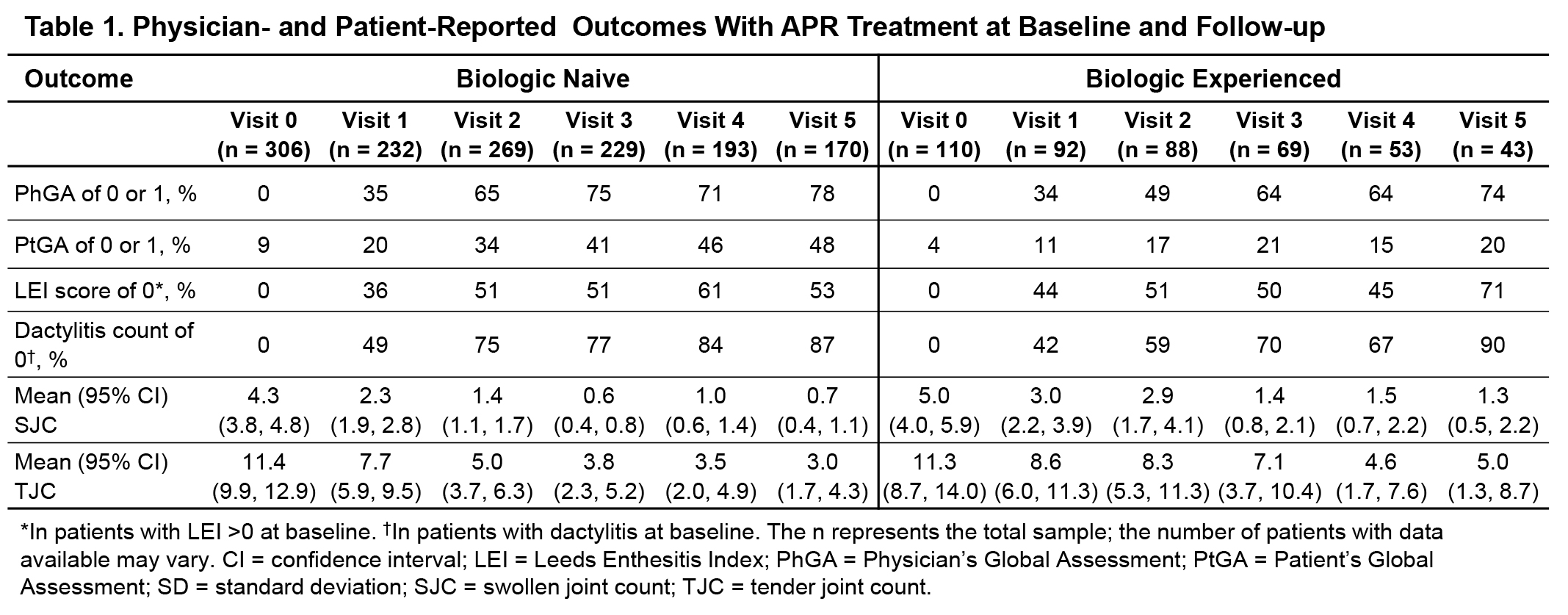
Results: Of the 418 patients who were enrolled in the full analysis set, 306 were biologic naive and 110 were biologic experienced. For biologic-naive vs biologic-experienced patients, mean (SD) age was 54.6 (11.0) vs 55.3 (10.6) years, mean (SD) body mass index was 29.5 (5.7) kg/m2 vs 29.6 (5.7) kg/m2, and 56.4% vs 65.1% were women. Biologic-naive vs biologic-experienced patients had similar baseline (mean [SD]) duration of psoriasis (24.9 [17.4] vs 27.7 [17.5] years), duration of PsA (17.0 [19.6] vs 19.7 [18.2] years), enthesitis counts (LEI count: 3.0 [1.7] vs 2.9 [1.7]), and dactylitis counts (2.3 [2.2] vs 2.0 [1.4]). During follow-up, early and sustained improvements were observed in PhGA, PtGA, enthesitis, dactylitis, SJC, and TJC assessments in both groups (Table 1); achievement of PtGA score of 0 or 1 was higher in biologic-naive vs biologic-experienced patients at all study visits (Table 1). Mean PsAID scores were lower for biologic-naive vs biologic-experienced patients at all study visits; biologic-naive patients achieved mean PsAID scores exceeding the Patient-Acceptable Symptom State (PASS; PsAID ≤4) as early as Visit 2 and maintained PsAID PASS up to Visit 5 (Figure 1). A greater proportion of patients in the biologic-naive vs biologic-experienced group achieved minimal disease activity (MDA) over visits (Figure 2). Rates of AEs commonly reported (≥5%) in APR clinical trials were similar in biologic-naive (n = 358) vs biologic-experienced (n = 128) patients in the safety analysis population of LAPIS-PsA (diarrhea: 31 [8.7%] vs 17 [13.3%]; nausea: 22 [6.1%] vs 8 [6.3%]; headache: 10 [2.8%] vs 5 [3.9%]; upper respiratory tract infection: 3 [0.8%] vs 1 [0.8%]).
Conclusion: In this real-world study of patients with PsA, APR demonstrated early and sustained effectiveness in patients with and without prior biologic therapy. More biologic-naive patients achieved PtGA 0-1 and PsAID PASS at all study visits vs biologic-experienced patients and were more likely to achieve early and sustained MDA. AEs were consistent with the known safety profile of APR.
To cite this abstract in AMA style:
Wollenhaupt J, Bach C, Roemmler-Zehrer J. Effectiveness and Safety of Apremilast in Biologic-Naive versus Biologic-Experienced Patients with Psoriatic Arthritis in Real-World Clinical Practice Settings in Germany: Interim Analysis of an Ongoing, Multicenter, Prospective, Non-interventional Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effectiveness-and-safety-of-apremilast-in-biologic-naive-versus-biologic-experienced-patients-with-psoriatic-arthritis-in-real-world-clinical-practice-settings-in-germany-interim-analysis-of-an-ongoi/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-apremilast-in-biologic-naive-versus-biologic-experienced-patients-with-psoriatic-arthritis-in-real-world-clinical-practice-settings-in-germany-interim-analysis-of-an-ongoi/