Session Information
Date: Friday, November 6, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Clinical Manifestations
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The Lupus Low Disease Activity State (LLDAS) has recently undergone prospective longitudinal validation in a multinational cohort, demonstrating the association of attaining LLDAS with protection from damage accrual and flare, results that have been replicated in numerous other observational cohorts. Domain 1 of the original LLDAS operational definition captured the absence of significant disease activity by requiring SLEDAI-2K <4, the absence of SLEDAI activity in major organs, and also the absence of haemolytic anaemia (HA) and gastrointestinal (GI) activity, manifestations not accounted for in the SLEDAI-2K. The requirement for absence of these infrequent clinical features creates the potential to misclassify patients’ LLDAS status, as there is no formal definition of HA and GI activity. This has in turn limited regulatory approval of LLDAS as a clinical trial endpoint. To determine the requirement for capture of HA and GI activity in the LLDAS definition we performed a sensitivity analysis.
Methods: We analysed the prospective LLDAS longitudinal validation dataset. To evaluate whether HA and GI activity were captured by the physician global assessment (PGA) criterion of LLDAS, we compared patients with and without HA and GI with respect to median PGA and the proportion of patients with PGA >1. The impact on subsequent damage accrual and flare of time-dependent associations of criterion 1 of the LLDAS definition, with and without inclusion of HA and GI activity, were assessed using Cox regression analysis.
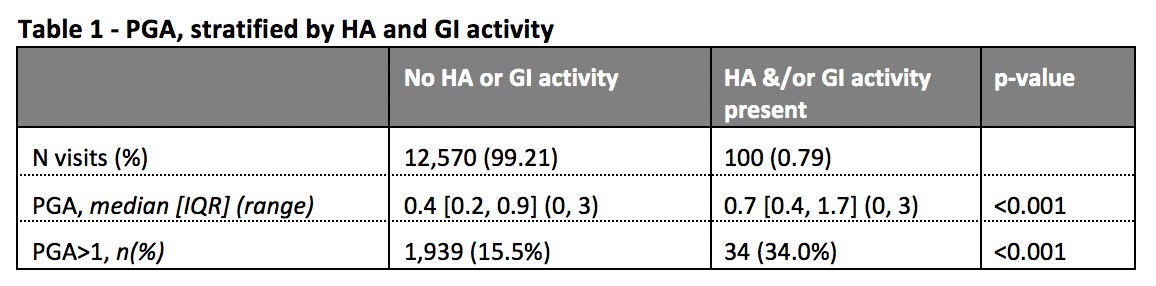
Results: Data on 1,707 patients, with 12,689 visits over 2.2 years were analysed. HA and GI activity were recorded in 28 and 73 visits, respectively. The median PGA, and the proportion of patients with PGA >1, were significantly higher in patients with either HA or GI activity (Table 1). Omitting the requirement for absence of HA and GI activity from criterion 1 of the LLDAS definition had no meaningful impact on the protective association with damage accrual in either visit by visit analysis (SLEDAI≤4 ignoring HA&GI: HR 0.55, 95% CI 0.43,0.70, p< 0.001; original definition: 0.55, 95% CI 0.43,0.71, p< 0.001). A similar lack of effect was observed for cumulative time effects of LLDAS (≥50% of time) on reducing damage accrual, and of LLDAS status on disease flare, when the requirement for absence of HA and GI activity was removed.
Conclusion: In data from a large prospective SLE cohort, the PGA adequately captures HA and GI activity. Removing the requirement for ‘absence of HA and GI activity’ in criterion 1 of the LLDAS definition is without effect on the protective associations of LLDAS attainment. Therefore, the definition of LLDAS does not require specification of HA and GI activity, improving its applicability to datasets where this information is not available and simplifying the use of LLDAS as a clinical trial endpoint. Together with published sensitivity analysis of the SLEDAI-2K and prednisolone dose cut-offs, this completes validation of the LLDAS endpoint.
To cite this abstract in AMA style:
Golder V, Kandane-Rathnayake R, Huq M, Louthrenoo W, Luo S, Wu Y, Lateef A, Sockalingam S, Navarra S, Zamora L, Hamijoyo L, Katsumata Y, Harigai M, Chan M, O'Neill S, Goldblatt F, Lau C, Li Z, Hoi A, Nikpour M, Morand E. Effect of Removing Haemolytic and Gastrointestinal Activity from the Operational Definition of the Lupus Low Disease Activity State – Implications for Use as a Trial Endpoint [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effect-of-removing-haemolytic-and-gastrointestinal-activity-from-the-operational-definition-of-the-lupus-low-disease-activity-state-implications-for-use-as-a-trial-endpoint/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-removing-haemolytic-and-gastrointestinal-activity-from-the-operational-definition-of-the-lupus-low-disease-activity-state-implications-for-use-as-a-trial-endpoint/