Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In the randomized, double-blinded, Phase 3 SELECT-COMPARE study, upadacitinib (UPA) + MTX demonstrated greater clinical and functional responses vs adalimumab (ADA) + MTX in patients with RA and inadequate response to MTX.1,2 In SELECT-COMPARE, patients with an insufficient response to their initial therapy were switched from UPA to ADA (and vice versa) according to treat-to-target (T2T) principles. To assess the effectiveness of such a strategy, we analyzed 1-year treatment outcomes in SELECT-COMPARE according to initial randomization group, regardless of whether or not patients subsequently switched therapy.
Methods: Patients initially randomized to UPA 15 mg once daily (QD) or ADA 40 mg every other week (EOW; both + MTX) for up to 48 weeks in SELECT-COMPARE were included in the analysis. As per the protocol-directed rescue strategy, patients experiencing < 20% improvement in tender or swollen joint counts at Week 14, 18, or 22, or Clinical Disease Activity Index (CDAI) >10 at Week 26, were switched from UPA to ADA or ADA to UPA in a blinded fashion. Efficacy outcomes included CDAI remission (≤2.8) and low disease activity (LDA; ≤10), DAS of 28 joints using CRP (DAS28[CRP]) < 2.6 and ≤3.2, and a composite of “deep response” (CDAI remission, HAQ-Disability Index < 0.5, and pain score < 20). Data are presented and attributed to initial randomized group (UPA or ADA) regardless of any subsequent switch in therapy. Time-averaged response rates were calculated as area under the curve of response rate standardized by 48 weeks. The proportions of patients who maintained Week 26 responses through 6 months of follow-up are also reported.
Results: This analysis included 651 patients initially randomized to UPA (of whom 245 switched to ADA) and 327 patients initially randomized to ADA (of whom 157 switched to UPA). Baseline characteristics including age, sex, and BMI were generally well balanced between randomized groups. At Week 48, similar proportions of patients initially randomized to UPA or ADA therapy achieved CDAI remission/LDA (27.6%/61.9% vs 24.8%/59.0%) and DAS28(CRP) < 2.6/≤3.2 (45.0%/60.2% vs 43.7%/59.0%) (Figure 1). However, a small but significantly greater proportion of patients achieved a deep response with initial UPA vs initial ADA therapy (17.8% vs 12.8%; p< 0.05). In addition, time-averaged response rates over 48 weeks were higher for initial UPA vs initial ADA therapy across efficacy outcomes (Figure 1). Similar trends were observed for other outcomes. Additionally, similar proportions of patients maintained Week 26 responses with initial UPA vs initial ADA therapy based on CDAI remission/LDA and DAS28(CRP) < 2.6/≤3.2 during 6-month follow-up (Figure 2).
Conclusion: Using a stringent T2T approach to RA management, rates of LDA or remission at 1 year were similar, regardless of whether patients were initially randomized to UPA or ADA. However, initial UPA therapy led to more frequent deep responses and higher time-averaged response rates vs initial ADA therapy.
- Fleischmann R, et al. Arthritis Rheumatol 2019;71:1788–800.
- Fleischmann R, et al. Ann Rheum Dis 2019;78:1454–62.
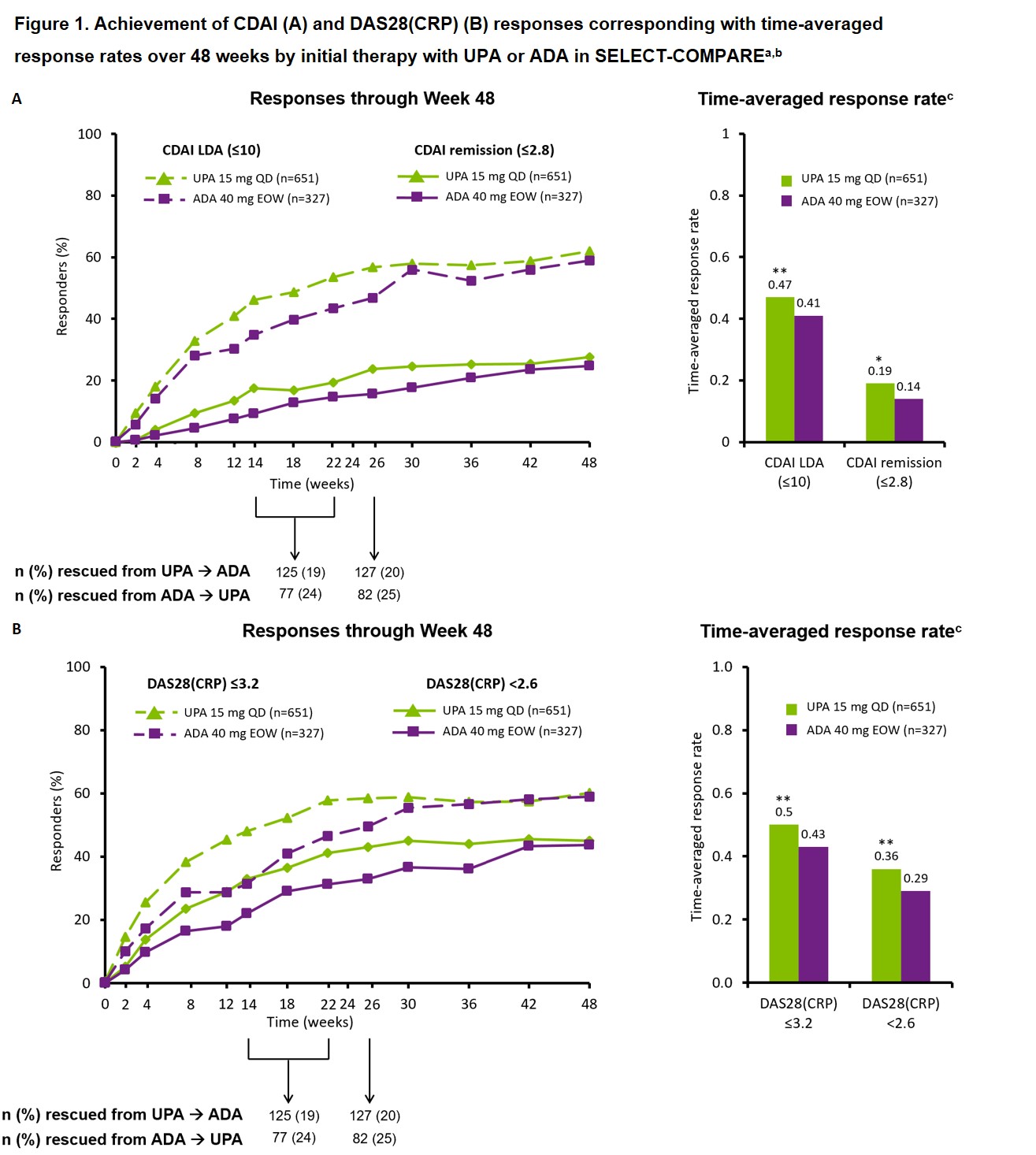 **p < 0.01, *p < 0.05 vs ADA. ᵃNon-responder imputation. ᵇBlinded rescue from UPA to ADA or ADA to UPA was permitted at Weeks 14, 18, and 22 for < 20% improvement in TJC/SJC and at Week 26 for CDAI < 10. Data are presented and attributed to original randomized group (UPA or ADA) regardless of any subsequent switch in therapy. ᶜCalculated as area under the curve of response rate standardized by length of study (48 weeks). ADA, adalimumab; CDAI, Clinical Disease Activity Index; DAS28(CRP), DAS of 28 joints using CRP; EOW, every other week; QD, once daily; SJC, swollen joint count; TJC, tender joint count; UPA, upadacitinib
**p < 0.01, *p < 0.05 vs ADA. ᵃNon-responder imputation. ᵇBlinded rescue from UPA to ADA or ADA to UPA was permitted at Weeks 14, 18, and 22 for < 20% improvement in TJC/SJC and at Week 26 for CDAI < 10. Data are presented and attributed to original randomized group (UPA or ADA) regardless of any subsequent switch in therapy. ᶜCalculated as area under the curve of response rate standardized by length of study (48 weeks). ADA, adalimumab; CDAI, Clinical Disease Activity Index; DAS28(CRP), DAS of 28 joints using CRP; EOW, every other week; QD, once daily; SJC, swollen joint count; TJC, tender joint count; UPA, upadacitinib
 ᵃAs observed. ᵇBlinded rescue from UPA to ADA or ADA to UPA was permitted at Weeks 14, 18, and 22 for patients with < 20% improvement in TJC or SJC and at Week 26 for patients with a CDAI < 10. Data are presented and attributed to original randomized group (UPA or ADA) regardless of any subsequent switch in therapy. ᶜMaintaining response defined as never losing response at any visit during ~6 months (22–26 weeks) follow up after first achieving response before or at Week 26. ADA, adalimumab; CDAI, Clinical Disease Activity Index; DAS28(CRP), DAS of 28 joints using CRP; EOW, every other week; QD, once daily; SJC, swollen joint count; TJC, tender joint count; UPA, upadacitinib
ᵃAs observed. ᵇBlinded rescue from UPA to ADA or ADA to UPA was permitted at Weeks 14, 18, and 22 for patients with < 20% improvement in TJC or SJC and at Week 26 for patients with a CDAI < 10. Data are presented and attributed to original randomized group (UPA or ADA) regardless of any subsequent switch in therapy. ᶜMaintaining response defined as never losing response at any visit during ~6 months (22–26 weeks) follow up after first achieving response before or at Week 26. ADA, adalimumab; CDAI, Clinical Disease Activity Index; DAS28(CRP), DAS of 28 joints using CRP; EOW, every other week; QD, once daily; SJC, swollen joint count; TJC, tender joint count; UPA, upadacitinib
To cite this abstract in AMA style:
Mysler E, Tanaka Y, Kavanaugh A, Aletaha D, Taylor P, Song I, Shaw T, Song Y, DeMasi R, Ali M, Fleischmann R. Impact of Upadacitinib or Adalimumab as Initial Therapy on the Achievement of 48-Week Treatment Goals in Patients with Rheumatoid Arthritis and Inadequate Response to Methotrexate: Post Hoc Analysis of a Phase 3 Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-upadacitinib-or-adalimumab-as-initial-therapy-on-the-achievement-of-48-week-treatment-goals-in-patients-with-rheumatoid-arthritis-and-inadequate-response-to-methotrexate-post-hoc-analysis-o/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-upadacitinib-or-adalimumab-as-initial-therapy-on-the-achievement-of-48-week-treatment-goals-in-patients-with-rheumatoid-arthritis-and-inadequate-response-to-methotrexate-post-hoc-analysis-o/
