Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Sarilumab is a human IL-6 receptor (IL-6R) inhibitor approved for the treatment of adults with moderate to severely active RA. In the TARGET study (NCT01709578), sarilumab significantly improved signs and symptoms of RA and physical function versus placebo in patients refractory to TNF inhibitors who were receiving background treatment with conventional synthetic DMARDs. Here we report the long-term safety and efficacy of subcutaneous (SC) sarilumab over 5 years in patients who continued treatment in the open-label extension (OLE) study, EXTEND (NCT01146652).
Methods: Patients who received double-blind placebo, sarilumab 150 mg, or sarilumab 200 mg every 2 weeks (q2w) in the 24-week randomized controlled trial TARGET were eligible to receive open-label sarilumab 200 mg q2w during EXTEND, with dose reduction to 150 mg q2w permitted to manage laboratory abnormalities or per investigator’s discretion. Safety outcomes are presented for the EXTEND population for the follow-up from TARGET baseline through EXTEND cut-off date (January 15, 2019). Efficacy assessments included Clinical Disease Activity Index (CDAI) score and the proportion of responders (CDAI ≤10 and ≤2.8) over time.
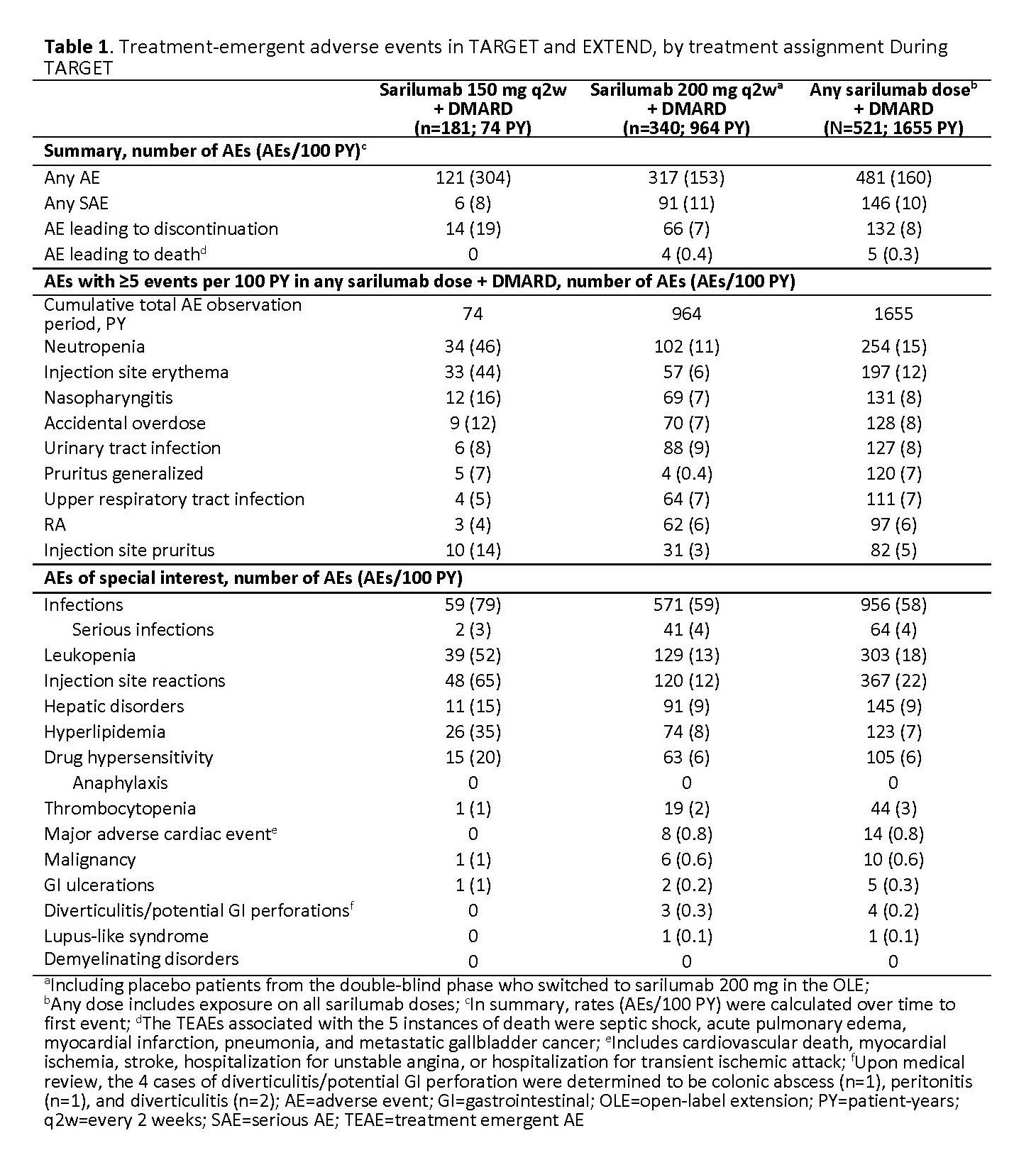
Results: Of the 546 patients randomized in TARGET, 454 (83%) entered EXTEND and were treated with open-label sarilumab 200 mg (original placebo group, n=156; sarilumab 150 mg, n=145; sarilumab 200 mg, n=153). At EXTEND baseline, demographic and clinical characteristics were similar between previous treatment groups from TARGET. Overall, 81% of patients in EXTEND were women, mean age was 53 years, and mean RA duration was 12 years. At the time of data cut-off, 199 (36%) patients had discontinued treatment, of whom 100 (18%) had discontinued due to treatment-emergent adverse events (AEs), 27 (5%) due to lack of efficacy, and 68 (13%) due to other reasons. Cumulative exposure to sarilumab through TARGET and EXTEND (n=521) was 1655 patient-years (PY), with 268 (51%) patients having ≥4 years’ exposure (Table 1). Overall, there were 160 TEAEs/100 PY, 10 serious AEs/100 PY, 8 AEs/100PY leading to discontinuation, and 0.3/100 PY leading to death (Table 1). The most common AEs were neutropenia (15/100 PY) and injection site erythema (12/100 PY; Table 1). Absolute neutrophil count < 1000 cells/mm3 (Grade 3–4 neutropenia) was observed in 74 (14%) patients, and normalized on treatment in 65% (48/74) of those patients. Alanine aminotransferase above 3-fold the upper limit of normal was observed in 46 patients (9%) and normalized on treatment in 54% (25/46) of those patients. Infections and serious infections occurred at rates of 58/100 PY and 4/100 PY, respectively. Clinical efficacy was sustained through 5 years of EXTEND (Table 2). At EXTEND Week 240 (n=95), mean ± SD change in CDAI score from TARGET baseline was −31 ± 15.
Conclusion: The safety profile of sarilumab was consistent with results of phase 3 trials, IL-6R inhibition, and SC administration, and no new safety signals were identified over 5 years of follow-up in patients with RA refractory to TNF inhibitors. Clinical efficacy was sustained through 5 years’ follow-up.
To cite this abstract in AMA style:
Fleischmann R, Maslova K, Leher H, Praestgaard A, Burmester G. Long-term Safety and Efficacy of Sarilumab over 5 Years in Patients with Rheumatoid Arthritis Refractory to Tumor Necrosis Factor Inhibitors [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-sarilumab-over-5-years-in-patients-with-rheumatoid-arthritis-refractory-to-tumor-necrosis-factor-inhibitors/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-sarilumab-over-5-years-in-patients-with-rheumatoid-arthritis-refractory-to-tumor-necrosis-factor-inhibitors/