Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: CT-P17 was developed as the first biosimilar of the high concentration (100 mg/mL), citrate-free formulation of reference adalimumab. The purpose of this study was to compare the pharmacokinetics (PK), safety, and immunogenicity of CT-P17 to EU-approved adalimumab (EU-adalimumab) and US-licensed adalimumab (US-adalimumab) up to Day 71 after a single subcutaneous (SC) injection of 40 mg (100 mg/mL) of each product in healthy subjects.
Methods: 312 healthy subjects aged 19 to 55 years were randomly assigned 1:1:1 to receive either CT-P17, EU-adalimumab or US-adalimumab. The primary objective was to evaluate PK equivalence based on area under the concentration-time curve (AUC) from time zero to infinity (AUC0-inf), AUC from time zero to the last quantifiable concentration (AUC0-last), and maximum serum concentration (Cmax). Secondary objectives were to assess additional PK parameters, safety, and immunogenicity.
Results: Demographics and baseline characteristics were well balanced among the 3 treatment groups. The 90% confidence intervals (CI) for the geometric least squares mean ratios of each of the primary PK parameters (AUC0–inf, AUC0–last, and Cmax) were within the predefined equivalence margin of 80% to 125% (Table 1). Secondary PK parameters (Tmax, t1/2, λz, CL/F, Vz/F, and %AUCextrap) were similar among the 3 treatment groups. Mean serum concentrations of adalimumab to Day 71 were comparable among the 3 treatment groups (Figure 1).
The overall safety profile was comparable among the 3 treatment groups (Table 2). The most frequently reported treatment-emergent adverse event (TEAE) was injection site reaction. Overall, most TEAEs were Grade 1 or 2 in intensity. There were 3 treatment-emergent serious adverse events (TESAEs), each of which was assessed as being unrelated to study drug.
Similar numbers of subjects among the 3 treatment groups tested positive for anti-drug antibodies (ADA) and neutralizing ADA (NAb). Overall, 99 (97.1%), 96 (94.1%) and 99 (95.2%) subjects in the CT-P17, US-adalimumab and EU-adalimumab groups, respectively, had ≥1 positive ADA result post-treatment and 79 (77.5%), 85 (83.3%) and 84 (80.8%) subjects in the CT-P17, US-adalimumab and EU-adalimumab groups, respectively, had ≥1 positive NAb result post-treatment. ADA titers were similar across the 3 treatment groups. AUC0–inf and AUC0–last each correlated negatively with ADA titer in all treatment groups (P-value < 0.0001; calculated using Fisher's z transformation). There was no statistically significant correlation of Cmax with ADA titer.
Conclusion: This study demonstrated PK equivalence of CT-P17 to the high concentration (100 mg/mL), citrate-free formulation of both US- and EU-sourced reference adalimumab in healthy subjects. Safety profiles, including immunogenicity, were comparable among the 3 treatment groups.
 Abbreviations: AUC0–inf, area under the concentration–time curve from time zero to infinity; AUC0–last, area under the concentration–time curve from time zero to the last quantifiable concentration; CI, confidence interval; Cmax, maximum serum concentration; EU adalimumab, European Union-approved adalimumab; gLSM, geometric least squares mean; US-adalimumab, United States-licensed adalimumab. *Ratio of geometric least squares mean and 90% CIs for the ratios
Abbreviations: AUC0–inf, area under the concentration–time curve from time zero to infinity; AUC0–last, area under the concentration–time curve from time zero to the last quantifiable concentration; CI, confidence interval; Cmax, maximum serum concentration; EU adalimumab, European Union-approved adalimumab; gLSM, geometric least squares mean; US-adalimumab, United States-licensed adalimumab. *Ratio of geometric least squares mean and 90% CIs for the ratios
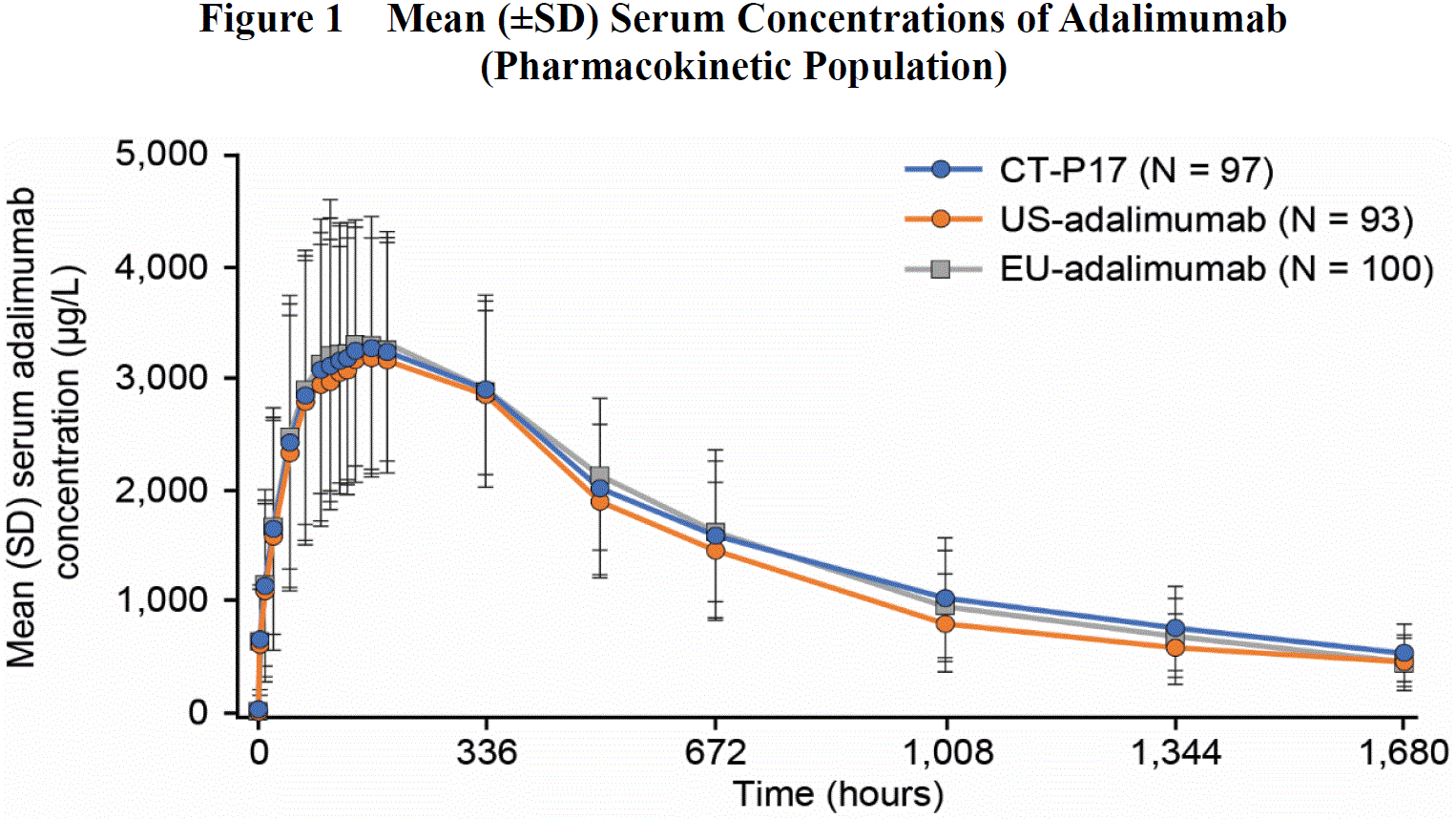 Abbreviations: EU-adalimumab, European Union-approved adalimumab; SD, standard deviation; US-adalimumab, United States-licensed adalimumab.
Abbreviations: EU-adalimumab, European Union-approved adalimumab; SD, standard deviation; US-adalimumab, United States-licensed adalimumab.
 Abbreviations: EU-adalimumab = European Union-approved adalimumab; TEAE = treatment-emergent adverse event; TESAE = treatment-emergent serious adverse event; US-adalimumab = United States-licensed adalimumab.
Abbreviations: EU-adalimumab = European Union-approved adalimumab; TEAE = treatment-emergent adverse event; TESAE = treatment-emergent serious adverse event; US-adalimumab = United States-licensed adalimumab.
To cite this abstract in AMA style:
Yu K, Jang I, Lim H, Hong J, Kim M, Park M, Kim A, Park M, Chung J, Ghim J, Lee S, Yoon S, Kwon I, Furst* D, Keystone E, Lee S, Kim S, Bae Y, Cha J, Kang H, Kay J. Pharmacokinetics and Safety of CT-P17, a Proposed High Concentration (100 mg/mL) Adalimumab Biosimilar, in Comparison with EU-Approved Adalimumab and US-Licensed Adalimumab; Results of a Phase 1, Randomized, Double-blind, Three-arm, Single-dose Study in Healthy Subjects [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/pharmacokinetics-and-safety-of-ct-p17-a-proposed-high-concentration-100-mg-ml-adalimumab-biosimilar-in-comparison-with-eu-approved-adalimumab-and-us-licensed-adalimumab-results-of-a-phase-1-rand/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-and-safety-of-ct-p17-a-proposed-high-concentration-100-mg-ml-adalimumab-biosimilar-in-comparison-with-eu-approved-adalimumab-and-us-licensed-adalimumab-results-of-a-phase-1-rand/
