Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Denosumab, a monoclonal antibody that binds to the receptor activator of nuclear factor kappa-B ligand (RANKL) is a popular choice for osteoporosis therapy. However, it has been demonstrated that late administration or discontinuation without change to a different osteoporosis therapy, results in a subsequent decline in bone mineral density (BMD) and the development of new vertebral fractures (often multiple). Recent guidance recommends that a repeat dose of denosumab or an alternative osteoporosis therapy is administered no later than 7 months after the prior denosumab dose. Therefore, the aim of the present study was to identify current practices regarding the timing of denosumab administration as well as subsequent treatment of osteoporosis after denosumab discontinuation at an academic medical center.
Methods: Pharmacy records for a single academic medical center were accessed to identify all dispenses of denosumab between October 1, 2017 and March 5, 2020. Individuals between 18 and 100 years of age, who had had at least one dose of denosumab dispensed were included, except for those whose denosumab was prescribed by an Oncologist or Urologist. The electronic medical record was then reviewed for age, specialty of the prescribing physician, total number and dates of denosumab injections. If denosumab was discontinued, it was recorded whether or not an alternative osteoporosis therapy was prescribed. T-tests and chi-squared tests were utilized to assess for any differences between those treated by Endocrinologists and Rheumatologists.
Results: 389 individuals were initially identified of whom 249 (92% female) met inclusion criteria (Table 1). Mean (SD) age was 75 (10) years. In 175 (70%) individuals, denosumab was prescribed by an Endocrinologist, compared to Rheumatology (45 individuals, 18%) and 29 (12%) by other specialties. The median number of doses of denosumab administered per individual was 3 (range 1-7) with the mean time between doses 6.4 months (SD 2.2 months). 446 (90%) of all subsequent denosumab doses were administered within 7 months. There was no significant difference in the mean time between doses nor the proportion of individuals receiving their denosumab dose within 7 months between Endocrinology and Rheumatology (p=0.5 and 0.09, respectively). For 99 (40%) individuals, greater than 7 months had elapsed since their last denosumab dose. Of those, only 7 (7%) were clearly transitioned to an alternative osteoporosis therapy, most commonly zolendronate (5 individuals).
Conclusion: 90% of denosumab doses were administered within the recommended timeframe of 7 months with no significant difference between Endocrinologists and Rheumatologists. However, where denosumab was discontinued, whether knowingly or not, only 7% were prescribed an alternative osteoporosis therapy. Given this, a considerable number of individuals may be at risk of decline in BMD and incident vertebral fracture. Moving forward, additional work is required to understand the reasons for both late administrations, but more importantly the lack of alternative therapy upon discontinuation before specific interventions can be implemented to address these deficiencies.
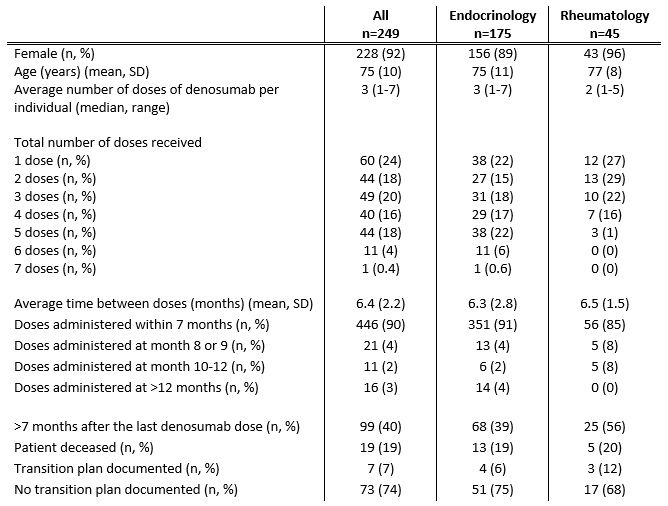 Table 1: Characteristics of Denosumab Prescribing
Table 1: Characteristics of Denosumab Prescribing
To cite this abstract in AMA style:
Raposo H, Lindstrom D, Cheah J. Appropriate Timing of Denosumab Dosing and Subsequent Therapy After Discontinuation: An Academic Medical Center Experience [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/appropriate-timing-of-denosumab-dosing-and-subsequent-therapy-after-discontinuation-an-academic-medical-center-experience/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/appropriate-timing-of-denosumab-dosing-and-subsequent-therapy-after-discontinuation-an-academic-medical-center-experience/
