Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate abaloparatide-induced changes in the bone formation indices of mineralizing surface (MS), bone formation rate (BFR) and mineral apposition rate (MAR); and to assess the effect of abaloparatide on 3 types of bone formation: modeling-based formation (MBF), remodeling-based formation (RBF), and overflow MBF (oMBF) in transiliac bone biopsies.
Methods: Twenty-three postmenopausal women with osteoporosis were enrolled at 4 centers in the US; all received open label abaloparatide (80 µg/day SC) for 3 months in this phase 3 study. Subjects were administered double fluorochrome labeling at baseline and prior to biopsy at 3 months. Bone formation indices were evaluated by histomorphometry. Changes at 3 months from baseline were assessed for the indices on cancellous, endocortical, intracortical and periosteal bone envelopes. Sites of bone formation were designated as MBF if the underlying cement line was smooth, RBF if scalloped, and oMBF if formed over smooth cement lines adjacent to scalloped reversal lines. Paired t-tests were used to compare the differences of the indices between baseline and 3 months in the 4 bone envelopes.
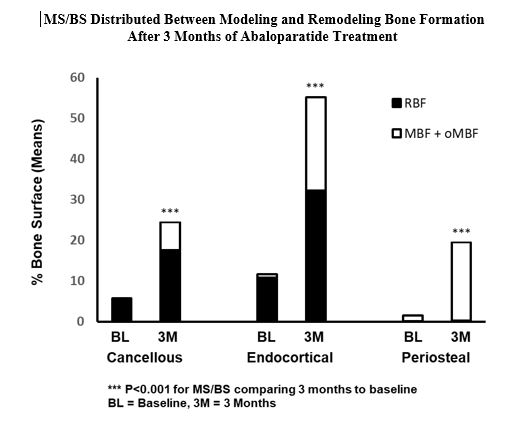
Results: Bone biopsies were obtained from 19 of the 23 subjects were enrolled in the study; all biopsies were evaluable. After 3 months of abaloparatide treatment, MS/ BS increased significantly from baseline in all of the 4 bone envelopes (p < 0.001), BFR/BS increased significantly in cancellous, endocortical and intracortical envelopes (p < 0.001); no significant change was observed in MAR in the 4 bone envelopes. Consistent with MS/BS, RBF/BS, MBF/BS and oMBF/BS increased significantly from baseline in the cancellous and endocortical envelopes (p < 0.001), as did MBF/BS in the periosteum (p < 0.001).
Conclusion: This study provides histomorphometric evidence that abaloparatide has a robust effect on bone formation in postmenopausal women with osteoporosis after 3 months of treatment. Both modeling- and remodeling-based bone formation were stimulated. This study also shows that abaloparatide has a favorable effect on cortical bone by increasing bone formation in endocortical and periosteal envelopes.
To cite this abstract in AMA style:
Dempster D, Zhou H, Rao S, Recknor C, Miller P, Leder B, Annett M, Ominsky M, Mitlak B. Effects of Abaloparatide on Modeling and Remodeling Based Bone Formation [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effects-of-abaloparatide-on-modeling-and-remodeling-based-bone-formation/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-abaloparatide-on-modeling-and-remodeling-based-bone-formation/