Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Date: Friday, May 1, 2020
Title: Poster Session 2
Session Type: ACR Abstract Session
Session Time: 5:00PM-6:00PM
Background/Purpose: Macrophage Activation Syndrome (MAS) and Secondary Hemophagocytic Lymphohistiocytosis (sHLH) are hyperinflammatory conditions caused by a cytokine storm. Prompt recognition and early treatment are essential to reduce the high disease mortality. Data in human and animals point to a pivotal role of IFN-γ in sHLH and in MAS in the context of systemic Juvenile Idiopathic Arthritis (sJIA).
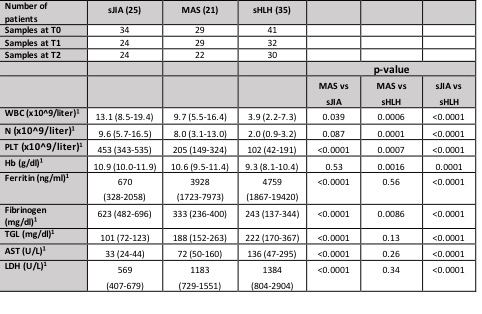
Methods: Routine laboratory parameters of disease activity and severity were collected from a cohort of patients with sHLH (n=35), MAS in the context of sJIA (n=21), and sJIA (n=25) at 3 different time points: active disease (T0), 7-10 days from starting therapy (T1) and in clinical inactive disease (1 to 3 months from onset) (T2). Serum levels of the IFN-γ related biomarkers (CXCL9, CXCL10 and Neopterin) were measured at each time point by ELISA.
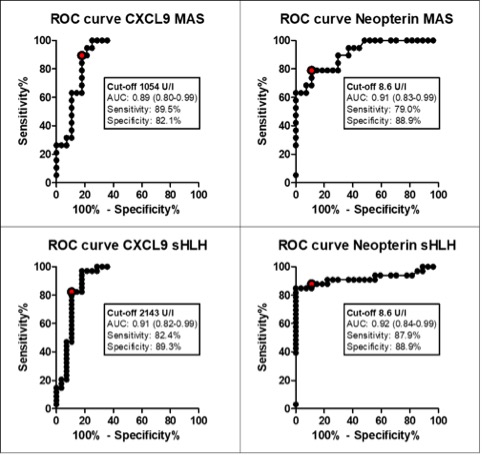
Results: A total of 265 samples were collected (data are detailed in Table 1). 33 (94%) patients with sHLH met the HLH-2004 diagnostic criteria, while 20 (95%) patients with MAS met the 2016 classification criteria for MAS. Fever was present in the majority of patients (93%), while hepatomegaly and splenomegaly were observed more frequently in MAS and sHLH patients (98% and 94% respectively) compared to sJIA (29.4% for both characteristics). Laboratory parameters at T0 are shown in Table 1. Using the 2016 classification criteria for MAS, we found that platelet count is a specific parameter, no patients with sJIA without MAS presented a value 684 mg/ml. Lactate dehydrogenase (LDH) levels are statistically higher in MAS and sHLH groups compared to sJIA. The ROC curve for LDH in MAS group shows an area under the curve (AUC= 0.89, p-value < 0.0001) with high sensitivity and specificity for a cut-off of 712 UI (79.3% and 79.4% respectively). CXCL9, CXCL10 and neopterin levels in T0 were significantly higher in MAS and sHLH patients compared to sJIA (Table 2). The ROC curves performed for each biomarker showed a statistically significant AUCs (p< 0.0001) and identified a cut off value for each biomarker in MAS and sHLH groups (Figure 1). CXCL9, CXCL10 and neopterin levels decreased progressively at T1 with a normalization in T2. CXCL9 decreased faster compared to neopterin, similarly to the decrease of the routine laboratory parameters.
Conclusion: Our results confirmed that platelet counts and ferritin are two relevant laboratory parameters, with high specificity and sensitivity respectively, to diagnose MAS in the context of sJIA. Even if LDH is not included in 2016 classification criteria for MAS in sJIA, we found that, in addition to the others, this parameter could help to discriminate MAS in sJIA. Moreover, our results confirmed that the IFN-γ related biomarkers are significantly increased in patients with MAS and sHLH and could be useful for diagnosis in addition to traditional laboratory parameters. Moreover, these biomarkers seem to be also useful to monitor clinical evolution and treatment response.
To cite this abstract in AMA style:
De Matteis A, Pires Marafon D, Caiello I, Pardeo M, Marucci G, Sacco E, Prencipe G, De Benedetti F, Bracaglia C. Traditional Laboratory Parameters and New Biomarkers in Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/traditional-laboratory-parameters-and-new-biomarkers-in-macrophage-activation-syndrome-and-secondary-hemophagocytic-lymphohistiocytosis/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/traditional-laboratory-parameters-and-new-biomarkers-in-macrophage-activation-syndrome-and-secondary-hemophagocytic-lymphohistiocytosis/