Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 4:00PM-6:00PM
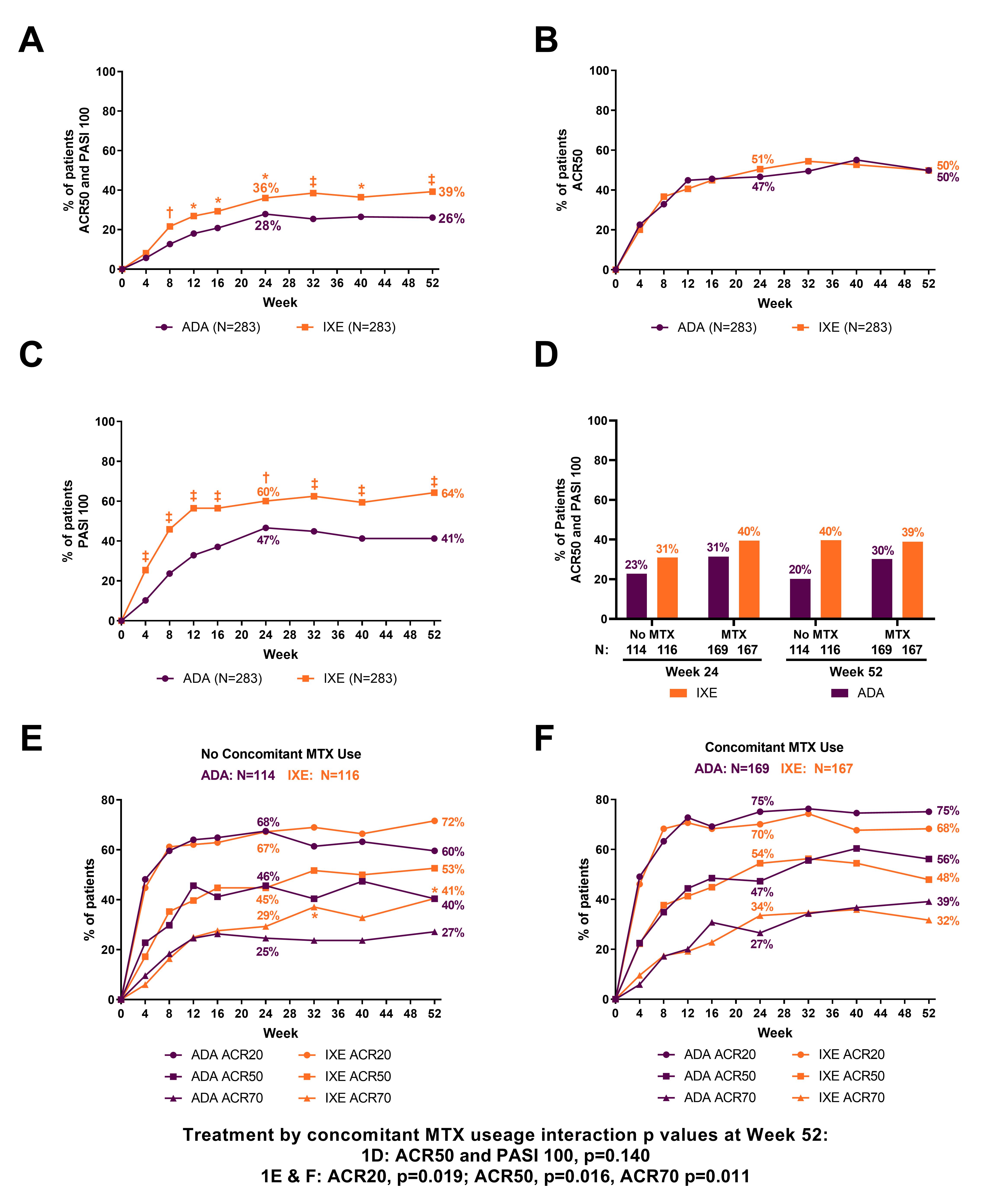
Background/Purpose: Multiple biologic DMARDs (bDMARDs) are available for treatment of PsA, but there are few direct comparisons of their efficacy and safety. Furthermore, efficacy of bDMARDs with or without concomitant MTX is one of the most clinically relevant questions for clinicians. Ixekizumab (IXE) was superior to adalimumab (ADA) at Week (Wk) 24 for simultaneous achievement of ACR50 and 100% improvement from baseline in the Psoriasis Area and Severity Index (PASI100) (primary endpoint) in patients (pts) with active PsA (SPIRIT-H2H) (Mease et al, Ann Rheum Dis 2019). We report final 52-wk efficacy and safety, and efficacy in subgroups defined by concomitant MTX use in SPIRIT-H2H.
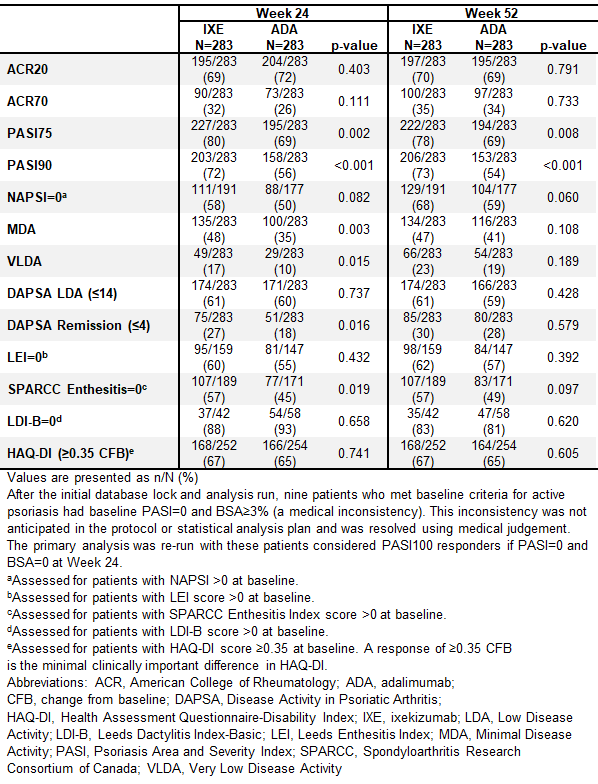
Methods: Pts with active PsA fulfilling Classification for Psoriatic Arthritis (CASPAR) criteria, ≥3/66 tender and ≥3/68 swollen joints, ≥3% psoriasis body surface area (BSA) involvement, no prior treatment with bDMARDs, and prior inadequate response to ≥1 conventional synthetic DMARD (csDMARD), were randomized 1:1 to open-label IXE or ADA (label dosing according to presence/absence of moderate-to-severe psoriasis [baseline BSA≥10%, PASI≥12, and static Physician’s Global Assessment≥3]) through 52 wks. Outcomes included the percentage of pts achieving both ACR50 + PASI100 simultaneously, ACR20/50/70, PASI75/90/100, Nail Psoriasis Severity Index (NAPSI)=0, Minimal Disease Activity (MDA), Very Low Disease Activity (VLDA, defined as MDA 7/7), Disease Activity in Psoriatic Arthritis Low Disease Activity (DAPSA LDA), DAPSA remission, Leeds Enthesitis Index (LEI)=0, Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index=0, Leeds Dactylitis Index-Basic (LDI-B)=0, and HAQ-Disability Index (HAQ-DI) ≥0.35 change from baseline. Efficacy was also analyzed in subgroups based on concomitant MTX. Efficacy outcomes were analyzed using logistic regression with nonresponder imputation for missing data. There were no adjustments for multiple comparisons. Safety outcomes are summarized for pts who received ≥1 dose of study treatment.
Results: Overall, 87% (246/283) and 84% (237/283) of pts randomized to IXE and ADA, respectively, completed Wk 52. IXE provided significantly greater response than ADA for simultaneous ACR50 + PASI100 through Wk 52 (Figure 1A). IXE performed at least as well as ADA at Wk 52 for all other outcomes (Figure 1B, 1C, Table 1). Simultaneous ACR50 + PASI100 response was numerically greater with IXE than ADA, regardless of concomitant MTX use (Figure 1D). MTX use by treatment interaction was significant for ACR20/50/70 at Wk 52 (Figure 1E-F). Treatment-emergent adverse events (AEs) occurred in 73.9% (IXE) and 68.6% (ADA) of pts. Serious AEs occurred in 4.2% (IXE) and 12.4% (ADA) of pts, and discontinuations due to AEs occurred in 4.2% (IXE) and 7.4% (ADA) of patients; no deaths occurred. (Table 2).
Conclusion: IXE provided significantly greater simultaneous joint and skin improvement versus ADA as early as Wk 8 and through Wk 52. IXE performed at least as well as ADA across multiple PsA domains including musculoskeletal and skin domains through Wk 52. Safety outcomes for IXE and ADA were consistent with their previously established safety profiles.
To cite this abstract in AMA style:
Smolen J, Nash P, Tahir H, Schulze-Koops H, Li L, Hojnik M, Gellett A, Liu-Leage S, Pillai S, Mease P. A Head-to-Head Comparison of Ixekizumab and Adalimumab in Biologic-Naïve Patients with Active Psoriatic Arthritis: Efficacy and Safety Outcomes from a Randomized, Open-Label, Blinded Assessor Study Through 52 Weeks [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-head-to-head-comparison-of-ixekizumab-and-adalimumab-in-biologic-naive-patients-with-active-psoriatic-arthritis-efficacy-and-safety-outcomes-from-a-randomized-open-label-blinded-assessor-study-th/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-head-to-head-comparison-of-ixekizumab-and-adalimumab-in-biologic-naive-patients-with-active-psoriatic-arthritis-efficacy-and-safety-outcomes-from-a-randomized-open-label-blinded-assessor-study-th/