Session Information
Date: Tuesday, November 12, 2019
Title: 5T090: Osteoarthritis – Clinical II: Novel Therapies (2756–2761)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: NSAIDs are associated with uncertain long-term benefits and significant toxicity in patients with knee osteoarthritis (OA). Our objective was to evaluate if discontinuing NSAIDs and engaging in a telephone-based cognitive behavioral therapy (CBT) program is non-inferior to continuing NSAIDs.
Methods: Patients in 4 medical centers, taking NSAIDs for knee OA pain on most days of the week for at least 3 months, were randomized to meloxicam or placebo for 4 weeks (blinded Phase 1). Those on meloxicam continued this medication for 10 weeks while those on placebo participated in a 10-week telephone-based CBT program (unblinded Phase 2). The primary outcome was the WOMAC pain score (LIKERT version) at 4-weeks. The minimum clinically important difference (MCID) is 2.1; the non-inferiority margin was set at 1. Secondary outcomes included time-averaged area under the curve (AUC) pain score at 4-weeks as well as AUC pain, WOMAC pain and disability scores, and global impression of change at end of Phase 2. Data were analyzed using linear regression models adjusted for baseline pain and site. We also estimated the incremental cost effectiveness ratio (ICER) of CBT compared to meloxicam at one year.
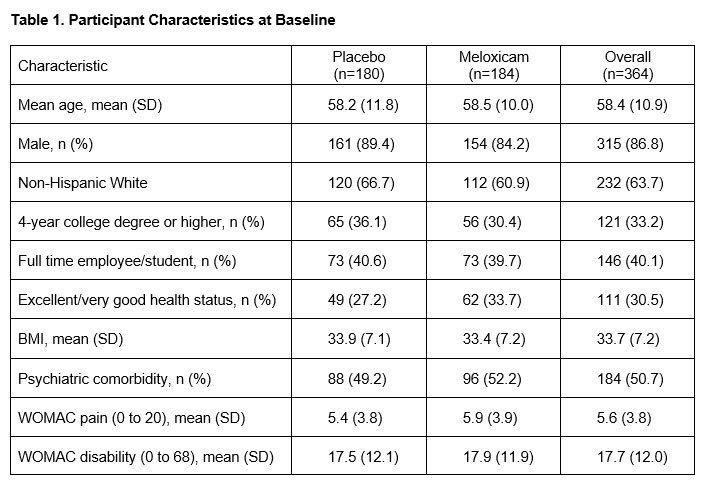
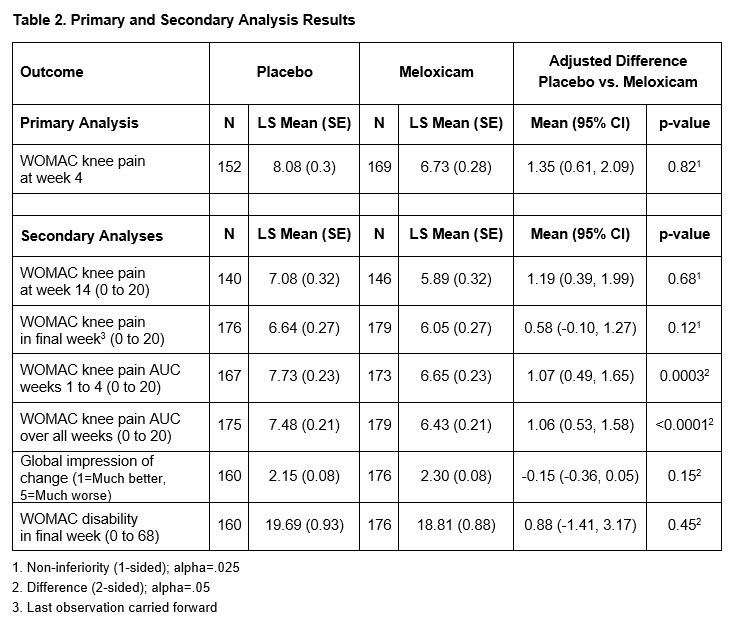
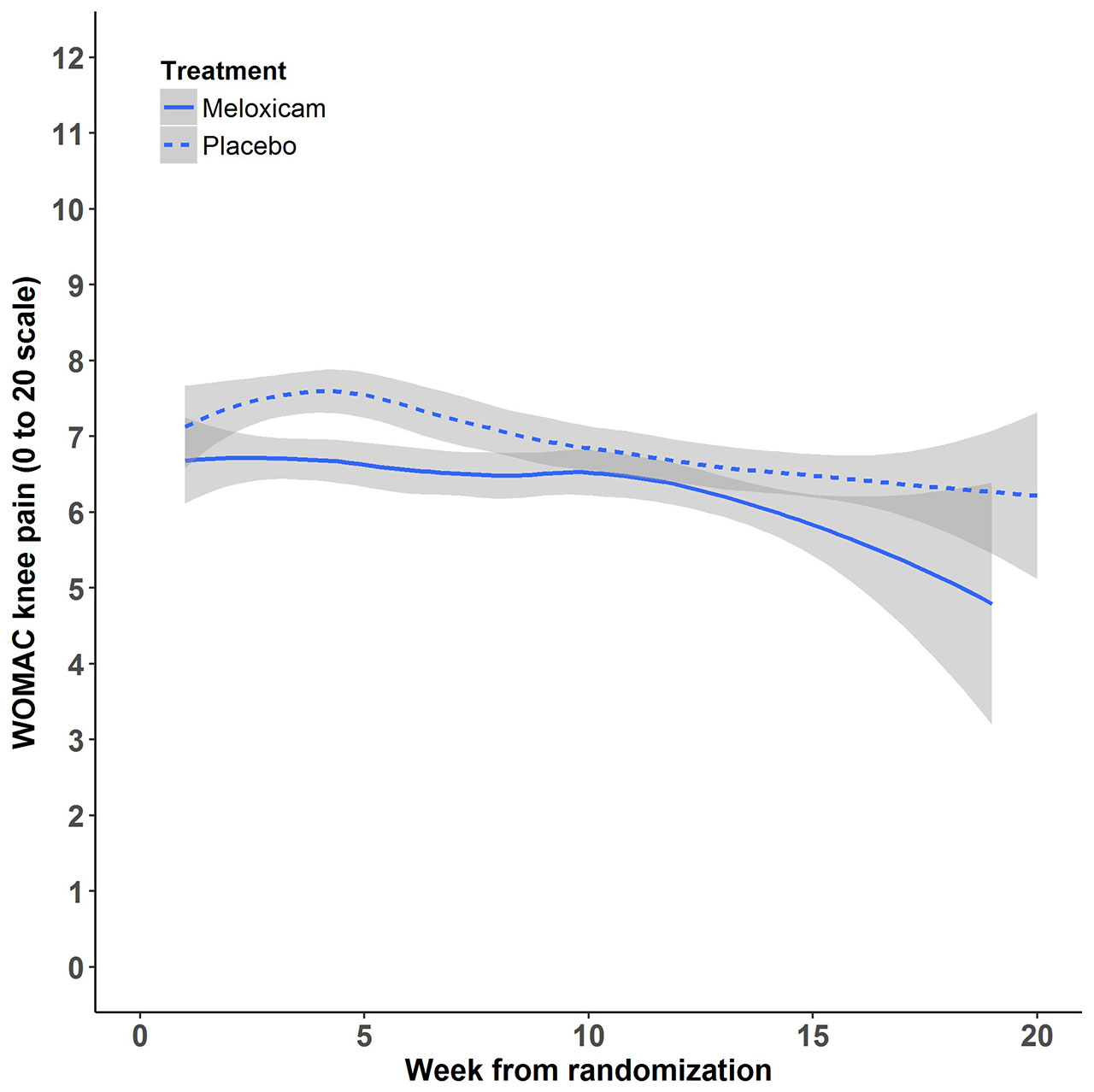
Results: 364 participants were randomized: 180 to placebo followed by CBT and 184 to meloxicam. Baseline demographic and clinical characteristics were well-balanced (Table 1). The overall mean pain at baseline was 5.6 (SD= 3.8). At 4 weeks, the raw mean pain score increased to 7.77 (SD= 4.01) in the placebo group and 6.75 (SD= 3.81) in the meloxicam group. After adjusting for baseline pain and site, the mean pain difference between placebo and meloxicam at 4 weeks was 1.35 (95% CI= 0.61 to 2.09, non-inferiority test p-value= 0.82). At week 14, the adjusted mean pain difference between placebo and meloxicam was 1.19 (95% CI= 0.39 to 1.99), non-inferiority p-value= 0.68). Mean pain scores over time (smoothed estimates) are illustrated in the Figure. There was no evidence of a difference in the global impression of change (p= 0.15) or lower extremity disability (p= 0.45) between the two groups at the end of Phase 2. Except for dyspepsia, which was more common in the meloxicam group (7.6% vs 0.6%), there were no significant differences in adverse events across groups. The ICER for CBT compared to continued meloxicam was $22,126.92 per quality-adjusted life year (QALY) gained. One-way sensitivity analyses of all input parameters across their plausible ranges showed that CBT remained cost-effective (ICERs < $100,000/QALY gained) compared to meloxicam.
Conclusion: Among patients with knee OA, we could not conclude that placebo and placebo followed by CBT is non-inferior to meloxicam within a non-inferiority margin of 1. However, the pain score differences between the two groups was small (less than the MCID of 2.1) and there was no difference in participants’ global impression of change or function at 14 weeks. Telephone-based CBT is cost effective compared to meloxicam.
To cite this abstract in AMA style:
Fraenkel L, Buta E, Goulet J, Brennan M, Heapy A, Suter L. Stopping NSAIDs for Arthritis Pain (SNAP): A Randomized Withdrawal Trial Comparing NSAIDs to Cognitive Behavioral Therapy [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/stopping-nsaids-for-arthritis-pain-snap-a-randomized-withdrawal-trial-comparing-nsaids-to-cognitive-behavioral-therapy/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/stopping-nsaids-for-arthritis-pain-snap-a-randomized-withdrawal-trial-comparing-nsaids-to-cognitive-behavioral-therapy/