Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: We propose categorizing lupus activity to better align with patient experience: Type 1 SLE activity includes classic symptoms such as arthritis and nephritis; Type 2 includes widespread pain, fatigue, depression, sleep disturbance, and perceived cognitive dysfunction. We previously found that only 53% of our patients with active Type 2 SLE received direct treatment for these symptoms, which is currently extrapolated from fibromyalgia treatment. We aimed to evaluate prescription of pharmacotherapies for Type 2 SLE symptoms one year after implementing the Type 1 and 2 categorization system.
Methods: This was a cross-sectional study of SLE patients (SLICC 2012 criteria) in a university lupus clinic from May 2018-March 2019. All patients completed Patient Health Questionnaire-9 (PHQ-9) and 2016 ACR Fibromyalgia criteria questionnaires. Active Type 2 SLE was defined as having a fibromyalgia severity score (FSS) > 10. We defined Type 2 medications as antidepressants (serotonin and norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressant (TCAs), mirtazapine, bupropion), muscle relaxers (cyclobenzaprine, tizanidine, baclofen, metaxalone), gabapentinoids (gabapentin, pregabalin), topiramate, zolpidem, trazodone, tramadol, anxiolytics, stimulants, and DHEA. Relationships between clinical variables and medications in different groups were analyzed using t-tests and Fisher’s exact test.
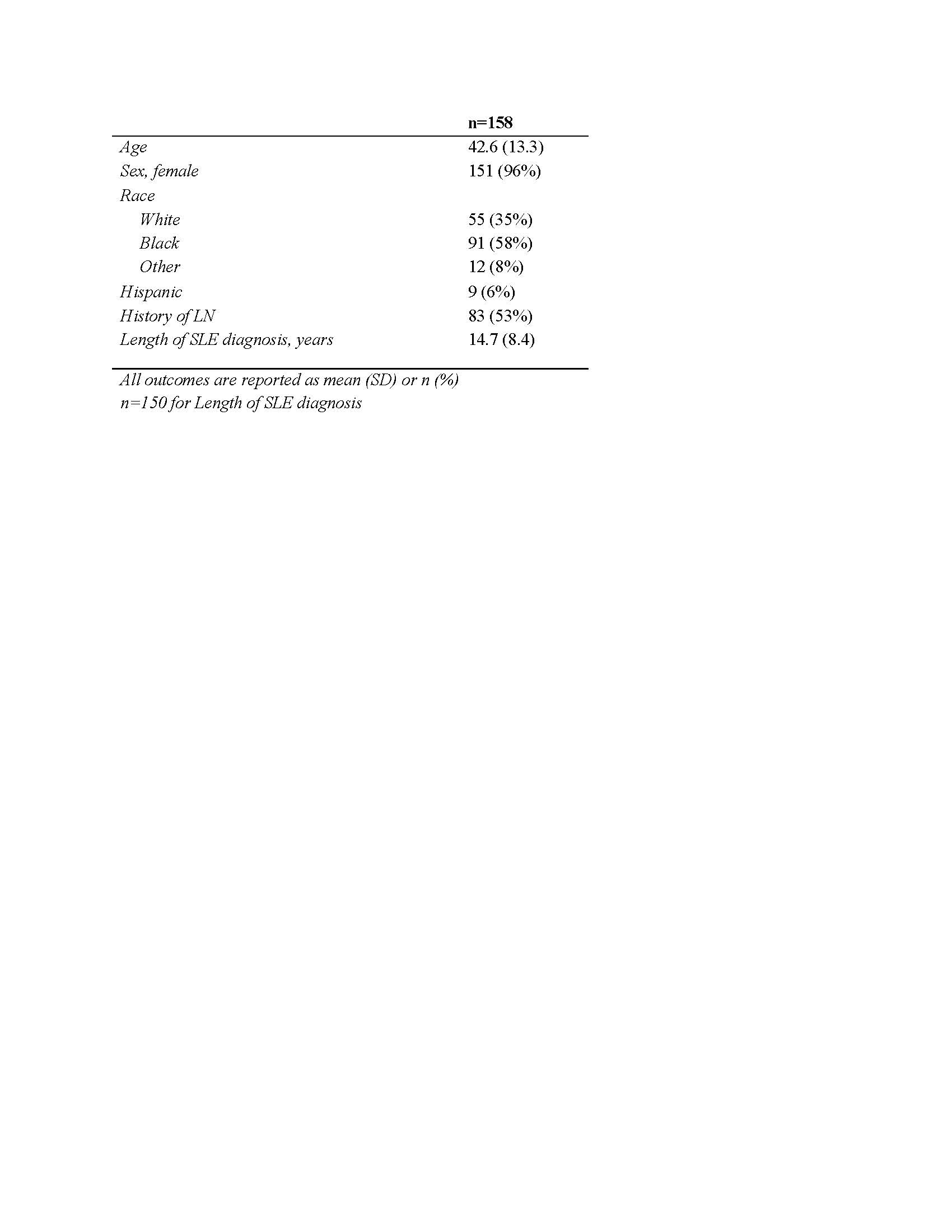
Results: Single-visit data from 158 patients were included (Table 1). There was a clear difference in prescribing patterns based on the presence or absence of Type 2 SLE: 70% of patients with active Type 2 SLE took at least one Type 2 medication compared to 40% of those without (Table 2). Medications differentially prescribed to patients with Type 2 activity included SNRIs (prescribed in 31% with Type 2 activity vs 6% without, p < 0.0001; primarily duloxetine), gabapentinoids (37% vs 13%, p = 0.001; primarily gabapentin), muscle relaxers (20% vs 6%, p = 0.01; primarily cyclobenzaprine), opioids (24% vs 10%, p = 0.02), trazodone (15% vs 4%, p = 0.02), and benzodiazepines (9% vs 1%, p = 0.02). There was no difference in prescriptions for SSRIs, NSAIDs, Vitamins D, or B12. Steroid use was higher in patients with active Type 1 symptoms (51% vs 33%) and average dose was higher (13 mg vs 7 mg). Among patients with active Type 2 SLE, there was no difference in Type 2 symptom severity between those who were taking corticosteroids and those who were not (Table 3).
Conclusion: After instituting formal assessments for Type 2 SLE activity, prescription of medications to address these symptoms has increased from 53% to 70%, aligning treatment patterns with patient symptomatology. Patients with active Type 2 SLE are differentially prescribed SNRIs, gabapentinoids, and muscle relaxers. Corticosteroids are more often prescribed to address active Type 1 rather than Type 2 activity and are not associated with Type 2 symptom severity. Together, these findings highlight the need for longitudinal studies to evaluate therapeutic efficacy of these medications and identify optimal pharmacologic and non-pharmacologic interventions for Type 2 SLE.
To cite this abstract in AMA style:
Whitney R, Eudy A, Clowse M, Criscione-Schreiber L, Doss J, Pisetsky D, Sun K, Sadun R, Rogers J. Pharmacotherapies Targeting Type 2 SLE Symptoms [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pharmacotherapies-targeting-type-2-sle-symptoms/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacotherapies-targeting-type-2-sle-symptoms/