Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate (MTX) is generally well tolerated in rheumatoid arthritis (RA) but little is known about the tolerability of MTX in psoriatic arthritis (PsA). One recent study has identified shorter MTX persistence among patients with PsA compared to RA in the US,1 while another study in Europe found similar persistence on MTX in RA and PsA.2 In addition, tolerability to MTX may be lower than tumor necrosis factor inhibitors (TNFi) in both PsA and RA. We hypothesized that shorter MTX persistence in patients with PsA is due to lower tolerability relative to RA patients.
Objective: To examine the relative reporting of side effects to MTX and TNFi among patients with PsA compared to RA within one year of drug initiation.
Methods: Retrospective cohort study conducted using data from the Forward, The National Databank for Rheumatic Diseases, from 2000 through 2018. Patients enroll in the registry and complete questionnaires every 6 months. Patients with RA and PsA were included in this study if they had at least one questionnaire completed prior to initiating a target drug (MTX or a TNFi) and at least one questionnaire within 12 months after initiation (new user design). The primary outcome of interest was the rate of reported side effects to the target drug. The prevalence of side effects over 1 year was reported among patients with RA and PsA, and stratified by use of MTX monotherapy, TNFi monotherapy, or MTX-TNFi combination therapy (starting either MTX or TNFi and adding second to regimen). In the primary analysis, patients could enter the TNFi cohort more than once if more than one drug was initiated. In a sensitivity analysis, we only allowed patients to enter the cohort for first TNFi.
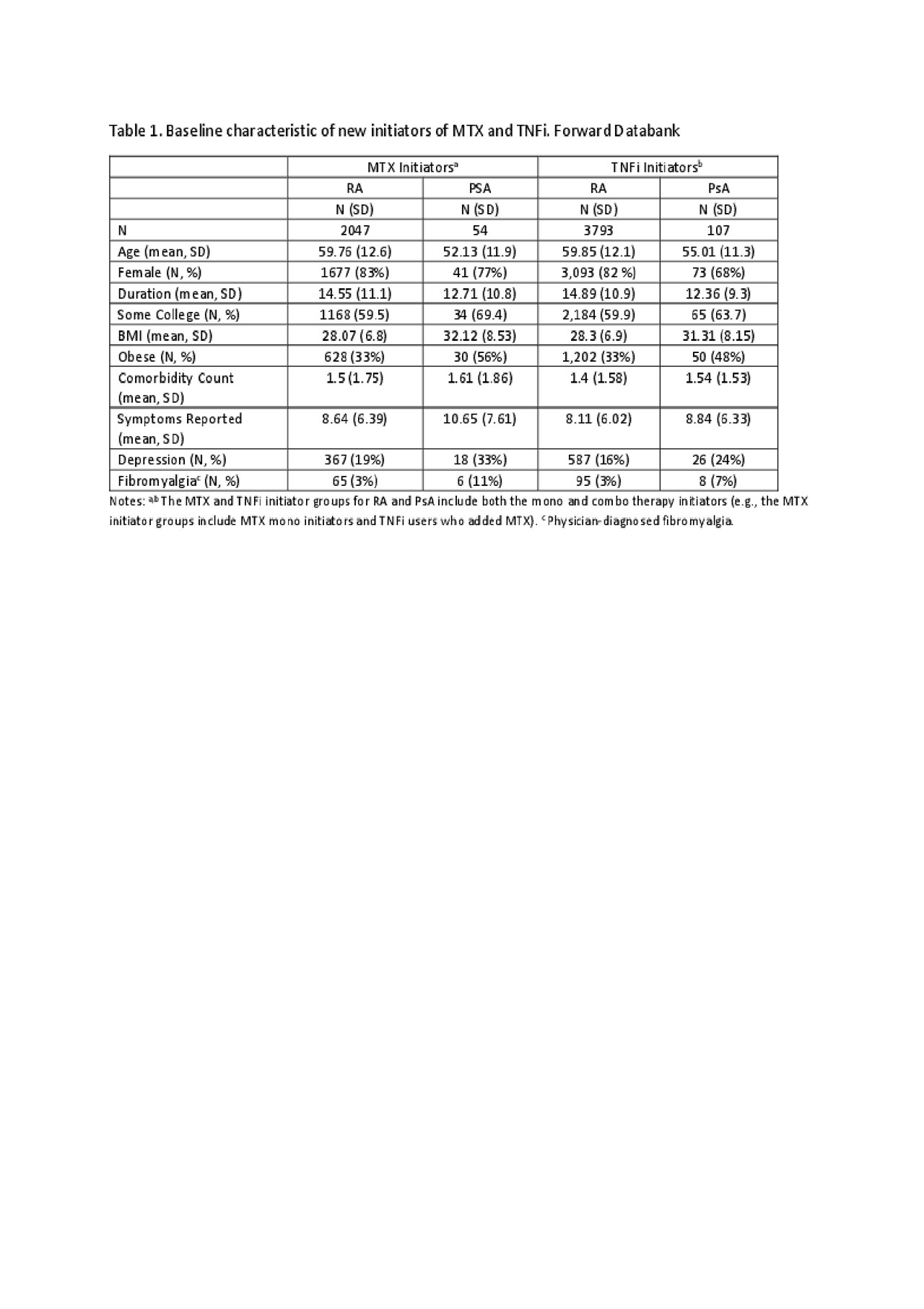
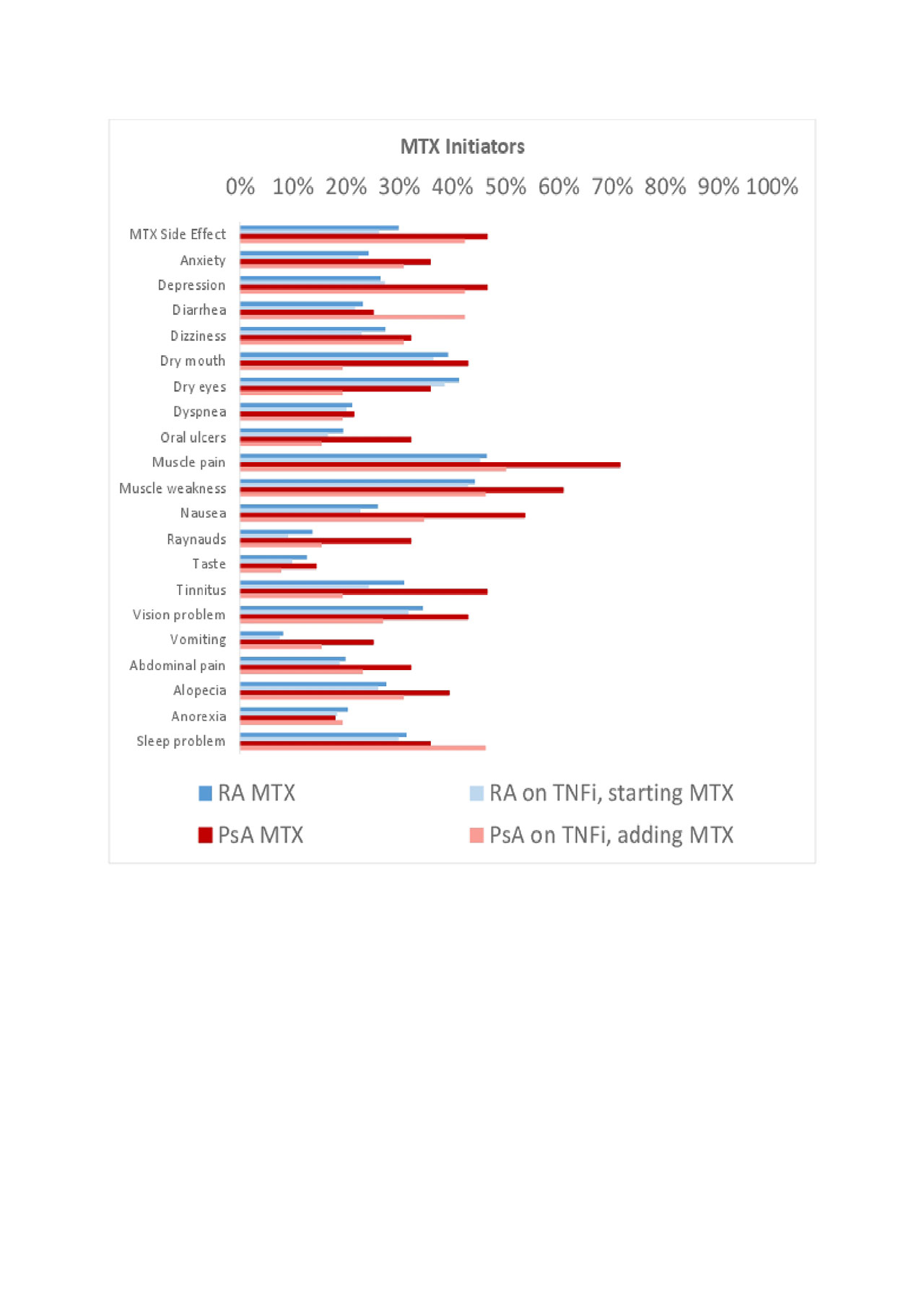
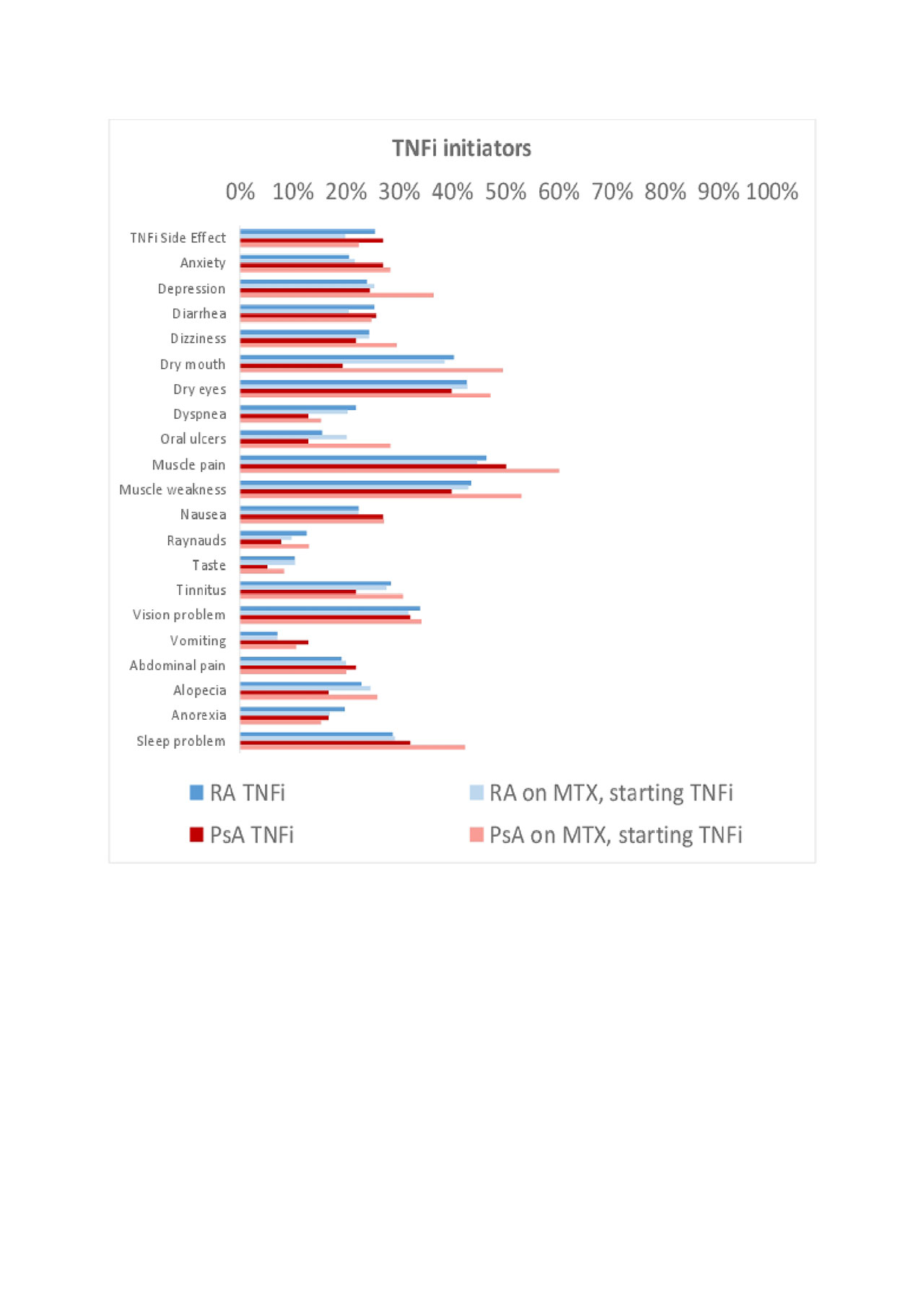
Results: Use of MTX or TNFi was reported among 11,571 patients with RA and 598 patients with PsA. Of these, 2,047 RA and 54 PsA patients were new initiators of MTX, and 3,793 RA and 107 PsA patients were new initiators of a TNFi. Baseline characteristics are shown in Table 1. Among initiators of MTX, 26-30% of RA patients compared to 42-46% of PsA patients reported a side effect in the first year (Figure 1). Among initiators of TNFi, 20-25% of RA and 22-27% of PsA patients reported a side effect in the first year (Figure 2). PsA patients initiating MTX were more likely to report nausea, vomiting, abdominal pain, anxiety, depression, tinnitus, and alopecia compared to RA patients initiating MTX or PsA patients initiating a TNFi.
Conclusion: Patients with PsA, in general, reported more symptoms than patients with RA. In particular, patients with PsA patients reported more side effects to MTX compared to patients with RA or patients with PsA initiating a TNFi.
References
- George, M.D., Baker, J.F. and Ogdie, A., Discontinuation of Methotrexate or TNF Inhibitors in Patients with Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis [abstract]. Arthritis Rheumatol 2018; (Vol. 70, suppl 10).
- Lie, E., van der Heijde, D., Uhlig, et al., Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Ann Rheum Dis, 2010; 69(4), pp.671-676.
To cite this abstract in AMA style:
Ogdie A, Maksabedian E, Stolshek B, Shaw Y, Michaud K. Side Effects of Methotrexate and TNFi: Differences in Tolerability Among Patients with PsA and RA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/side-effects-of-methotrexate-and-tnfi-differences-in-tolerability-among-patients-with-psa-and-ra/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/side-effects-of-methotrexate-and-tnfi-differences-in-tolerability-among-patients-with-psa-and-ra/