Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Our aim was to develop recommendations for the management of methotrexate (MTX) when considering the combination with biological (b) or targeted synthetic (ts) disease modifying drugs (DMARDs) in rheumatoid arthritis (RA).
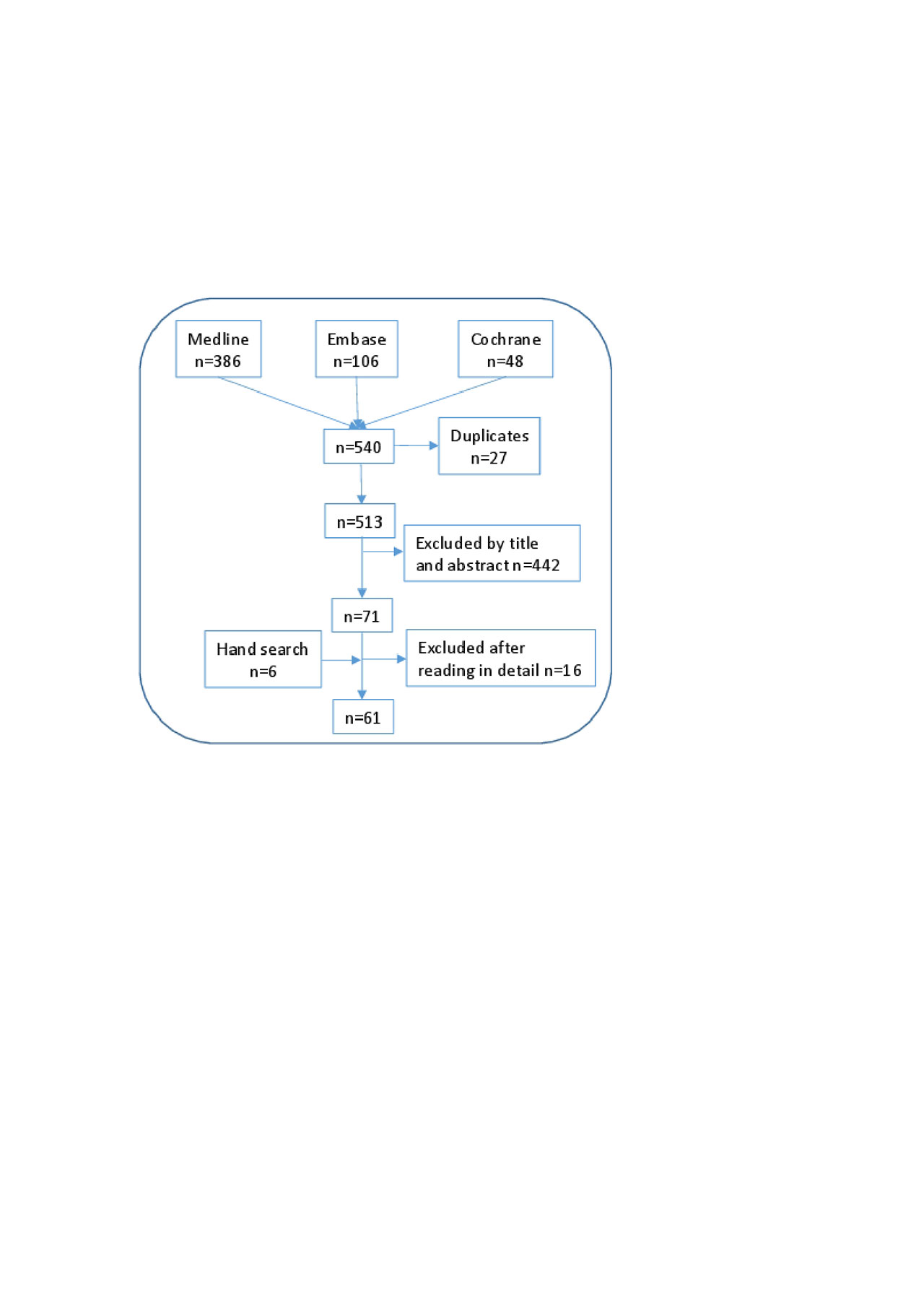
Methods: Methodological procedures included nominal discussion group, systematic literature review, and Delphi survey for agreement. A panel of 11 expert rheumatologists, including two coordinators, was selected. The coordinators defined the goals, scope and users of the document and delivered a set of 11 relevant clinical questions regarding the use of MTX in combination with b or tsDMARDs, including the indication, dose, route of administration, dose-adjustments, efficacy and safety. To address them, an extensive systematic literature review was performed. The following PICO queries and inclusion criteria were defined, 1) RA patients (population); 2) on, or considering the start of combined therapy with MTX and b or tsDMARDs (intervention); reporting efficacy and/or safety variables like composite activity indexes, radiographic progression, serious adverse events, etc. (outcomes); 3) searches restricted to systematic literature reviews and meta-analysis, humans, English and Spanish articles. Then, an expert documentalist designed the search strategies (Medline, Embase and the Cochrane Library up to January 2019), using Mesh ant text word terms. Two reviewers selected the articles and collected data, independently. Subsequently, a manual search of the bibliography of the articles that were finally included was performed. Evidence tables were produced. The quality was evaluated with the Oxford Center for Evidence Based Medicine recommendations. Simultaneously, EULAR and ACR abstracts (2017 and 2018) were evaluated, along with national and international consensus and guidelines. This information was used by the coordinators to generate preliminary recommendations. Then, all the experts, in a nominal discussion group, agreed on the proposed objectives, scope and users, and analyzed the results of the review and preliminary recommendations. Recommendations were re-formulated as considered, and voted in a Delphi process (yes/no). Agreement was considered if at least 80% of experts voted yes. The level of evidence and grade or recommendation were assigned using the Oxford Centre for Evidence-Based Medicine guidance. The full document was critically appraised by the experts and the project was supervised at all steps by a methodologist.
Results: The systematic literature search retrieved 513 citations of which 61 were finally included. A total of 9 preliminary recommendations were proposed, 2 were not voted and were explained in the main text of the document, 3 new ones were proposed and 10 were finally voted and accepted (depicted in table 1 with their level of evidence, grade of recommendation and grade of agreement). The level of agreement was very high in all of them and was achieved in the first Delphi round.
Conclusion: This document is intended to help clinicians solve usual clinical questions and facilitate decision making when treating RA patients with MTX in combination with bDMARDs or tsDMARDs.
To cite this abstract in AMA style:
Tornero J, Alperi-López M, Castellvi I, de Agustín de Oro J, Escudero A, Garcia-Vicuña R, González-Gay M, Hidalgo C, Rubio E, Sanmarti R, Casamira N, Calvo-Alen J. Consensus Statement and Recommendations on Methotrexate Use in Combined Therapy with Biological or Targeted Synthetic Disease Modifying Drugs in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/consensus-statement-and-recommendations-on-methotrexate-use-in-combined-therapy-with-biological-or-targeted-synthetic-disease-modifying-drugs-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consensus-statement-and-recommendations-on-methotrexate-use-in-combined-therapy-with-biological-or-targeted-synthetic-disease-modifying-drugs-in-patients-with-rheumatoid-arthritis/