Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with RA experienced an increase in the whole-body insulin sensitivity and a reduction in HbA1c levels from treatment with abatacept, which is a type of targeted DMARD (tDMARD).1 Abatacept is often used in tumor necrosis factor inhibitor (TNFi)-experienced patients; however, comparative impact of tDMARDs on type 2 diabetes mellitus (T2DM) related outcomes is lacking. This study examined healthcare resource utilization (HCRU) and costs associated with T2DM in TNFi-experienced patients with RA and T2DM who switched to another tDMARD.
Methods: In this retrospective cohort study, 100% Medicare fee-for-service (FFS) claims (Parts A/B/D) were used to identify TNFi-experienced RA patients who initiated abatacept, another TNFi (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab) or non-TNFi (anakinra, rituximab, sarilumab, tocilizumab, tofacitinib) from 2010 through 2017. Patients ≥65 years of age were included if they had ≥2 diagnosis of RA, ≥1 diagnoses of T2DM or ≥1 T2DM medications, no recent history of cancer, and were continuously enrolled for 12 months pre-index date or date of tDMARD initiation. Patients were followed from tDMARD initiation until discontinuation of the index treatment, disenrollment, death, or end of study period, whichever occurred first. Patients who switched to abatacept were propensity score (PS) matched to TNFi and non-TNFi users separately on a wide range of confounders. T2DM-related complications for which HCRU and costs were estimated were from the validated Diabetes Severity Complication Index (DCSI)2 – neuropathy, nephropathy, cerebrovascular, cardiovascular, retinopathy, peripheral vascular disease and metabolic. The HCRU of T2DM-related complications was estimated per 1,000 patients per month using counts of inpatient stay, ER visits and outpatient visits whereas Medicare costs or payments (2019 USD) were estimated as per-patient per month (PPPM).
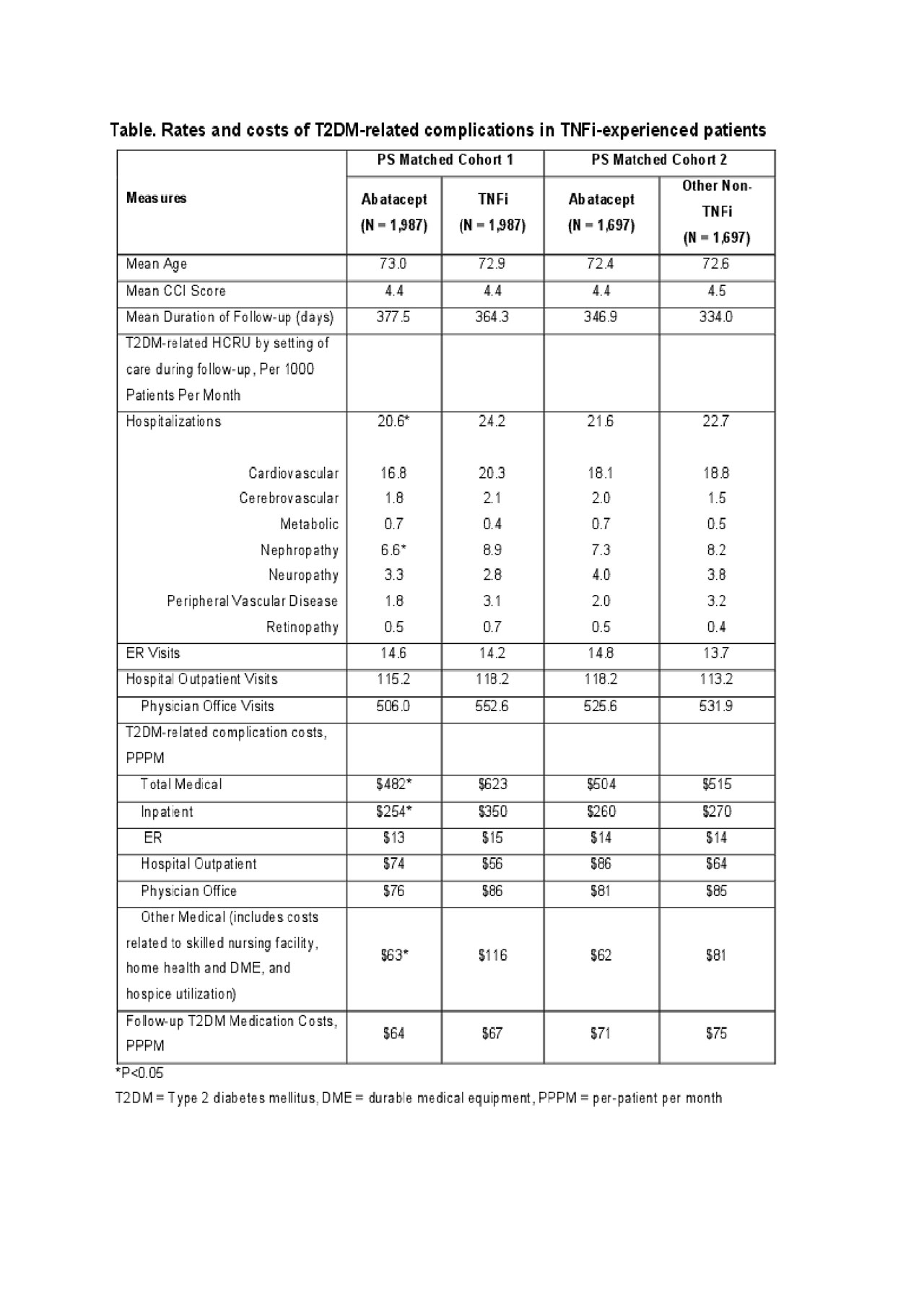
Results: A total of 1,987 PS-matched pairs of abatacept and TNFi initiators, and 1,697 PS-matched pairs of abatacept and non-TNFi initiators were identified. During follow-up, the rate of hospitalizations with T2DM-related complications per 1,000 patients per month was the lowest in abatacept compared to both TNFi (20.6 vs. 24.2) and other non-TNFi groups (21.6 vs. 22.7). PPPM T2DM-related complication costs were lower in abatacept than in TNFI’s and non-TNFI users by $141 and $11 respectively. The major driver of healthcare costs for each group was the utilization of inpatient services. PPPM T2DM-related costs decreased following initiation of the new line of treatment for both abatacept (12%-21%) and non-TNFI (14%) initiators, but it was the opposite for initiators of TNFis (10% increase).
Conclusion: These findings suggest that TNFi-experienced Medicare patients with RA and T2DM who switch to abatacept had a reduction in the rate and cost of hospitalizations associated with T2DM-related complications in comparison to patients who switch to other tDMARDs.
References:
- Ursini F, et al. Medicine (Baltimore). 2015;94(21):e888.
- Glasheen WP, et al. J Diabetes Complications. 2017;31(6):1007-1013.
To cite this abstract in AMA style:
Patel V, Pulungan Z, Shah A, Jones B, Petrilla A, Ferri L, Han X, Michaud K. Comparison of Healthcare Resource Utilization (HCRU) and Costs of Type 2 Diabetes Mellitus (T2DM)-Related Complications in TNFi-Experienced Medicare Beneficiaries with Rheumatoid Arthritis (RA) and T2DM Who Switch to Abatacept or Other Targeted Disease-Modifying Anti-Rheumatic Drugs [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparison-of-healthcare-resource-utilization-hcru-and-costs-of-type-2-diabetes-mellitus-t2dm-related-complications-in-tnfi-experienced-medicare-beneficiaries-with-rheumatoid-arthritis-ra-and-t2/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-healthcare-resource-utilization-hcru-and-costs-of-type-2-diabetes-mellitus-t2dm-related-complications-in-tnfi-experienced-medicare-beneficiaries-with-rheumatoid-arthritis-ra-and-t2/