Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) patients have a high prevalence of cardiovascular comorbidities including diabetes mellitus (DM). Past studies have suggested a potential beneficial effect of abatacept on insulin sensitivity, but the effect of biologic therapy on DM severity in RA patients with DM is generally unknown. The objective of this study was to compare the rates of DM treatment switching or intensification among patients with RA and DM (type 1 or 2), newly initiating abatacept versus other disease-modifying antirheumatic drugs (DMARD).
Methods: We identified RA patients aged ≥18 years with ≥2 RA diagnoses separated by 7-365 days using claims data from Truven MarketScan database (2005-2016). We included new users of abatacept, tumor necrosis factor inhibitors (TNFi) (adalimumab, etanercept, certolizumab, golimumab, and infliximab), rituximab, tocilizumab, and tofacitinib. The date of their 1st drug dispensing was defined as the index date. We required >365 days of continuous enrollment prior to the index date, defined as the baseline period. We excluded patients with history of malignancy. Among these RA patients, we identified patients with DM by using >1 diagnosis for either type 1 or type 2 DM and >1 anti-diabetic drug prescription during baseline. The primary outcome was ‘DM treatment switching or intensification’ defined as adding or switching to a different oral antidiabetic medication or insulin. We calculated incidence rates (IR) and hazard ratio (HR) of DM treatment switching or intensification in patients initiating abatacept versus other biologic DMARDs or tofacitinib.
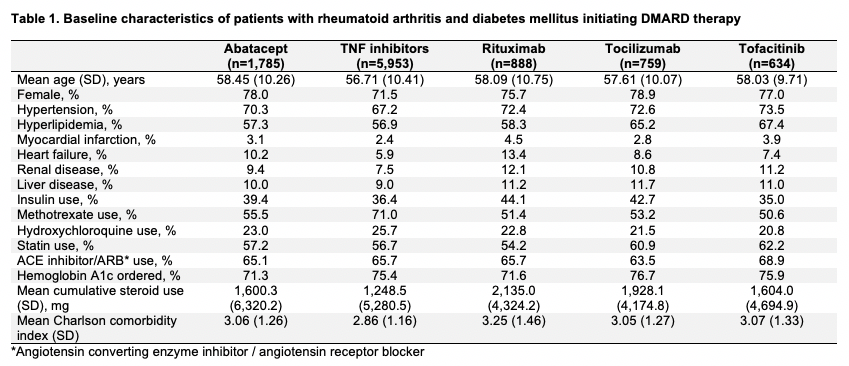
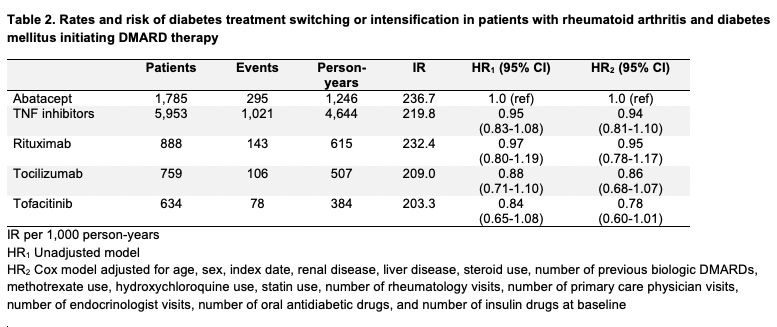
Results: We included 10,019 patients with both RA and DM initiating abatacept (mean age 58.5 years, female 78%), TNFi (56.7, 71%), rituximab (58.1, 76%), tocilizumab (57.6, 79%), or tofacitinib (58.0, 77%) (Table 1). Cardiovascular comorbidities were prevalent; hypertension was present in up to 74% and hyperlipidemia was present in up to 67% of patients. Baseline insulin use was highest in the rituximab group (44%) and lowest in the tofacitinib group (35%). Over the 7,396 total person-years of follow up, there were 1,643 total DM treatment switching or intensification events (Table 2). The crude IR of DM treatment switching or intensification per 1,000 person-years was highest in abatacept initiators (IR 236.7) and lowest in tofacitinib initiators (IR 203.3). After adjusting for 15 baseline covariates, the risk of DM treatment switching or intensification was similar between abatacept and TNFi (HR 0.94, 95% confidence interval [CI] 0.81-1.10), rituximab (HR 0.95, 95% CI 0.78-1.17), and tocilizumab (HR 0.86, 95% CI 0.68-1.07) and lower for tofacitinib (HR 0.78, 95% CI 0.60-1.01).
Conclusion: In patients with both RA and DM, we found no difference in the risk of DM treatment switching or intensification after initiating abatacept versus TNFi, rituximab, and tocilizumab, while the risk appeared to be lower for tofacitinib initiators compared to abatacept.
To cite this abstract in AMA style:
Chen S, Lee H, Jin Y, Liu J, Kim S. Risk of Diabetes Treatment Switching or Intensification Associated with Use of Abatacept versus Other Biologic Drugs in Patients with Rheumatoid Arthritis and Diabetes Mellitus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-of-diabetes-treatment-switching-or-intensification-associated-with-use-of-abatacept-versus-other-biologic-drugs-in-patients-with-rheumatoid-arthritis-and-diabetes-mellitus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-diabetes-treatment-switching-or-intensification-associated-with-use-of-abatacept-versus-other-biologic-drugs-in-patients-with-rheumatoid-arthritis-and-diabetes-mellitus/