Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Some concerns have been raised about subsequent severe acute localized reactions (SALR) (flares, pseudoseptic reactions, etc.) following hyaluronic acid (HA) injections for pain relief of knee osteoarthritis (OA). SALR has been speculated to be possibly related to the crosslink of hylan or an allergic reaction to hyaluronan of avian origin, but similar reactions have been reported following the use of non-crosslinked, non-animal, and/or naturally derived HA. We compared surrogate SALR measures between hylan G-F 20 and non-hylan G-F 20 patients and evaluated the corresponding risk factors for hylan G-F 20 patients in a real-world setting.
Methods: Knee OA patients were identified from the Optum Clinformatics (UnitedHealth Group private payer) dataset (Jan 2006-June 2016) and stratified into hylan G-F 20 and non-hylan G-F 20 users. The occurrences of surrogate SALR measures including inflammation/infection, corticosteroid (CS) injections, arthrocentesis/aspiration, and office visits were evaluated within three days of HA use. Logistic regression was used to evaluate risk factors.
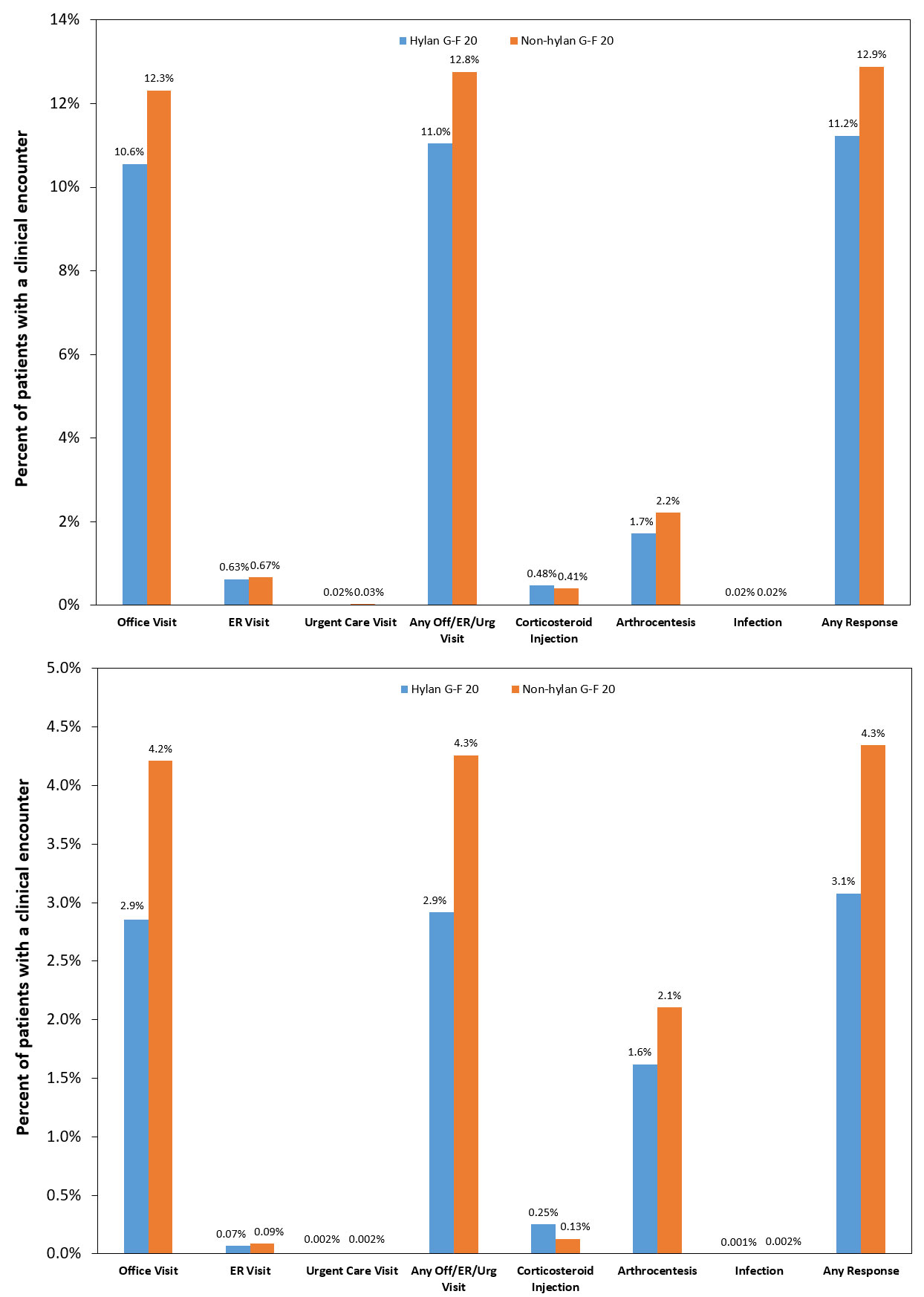
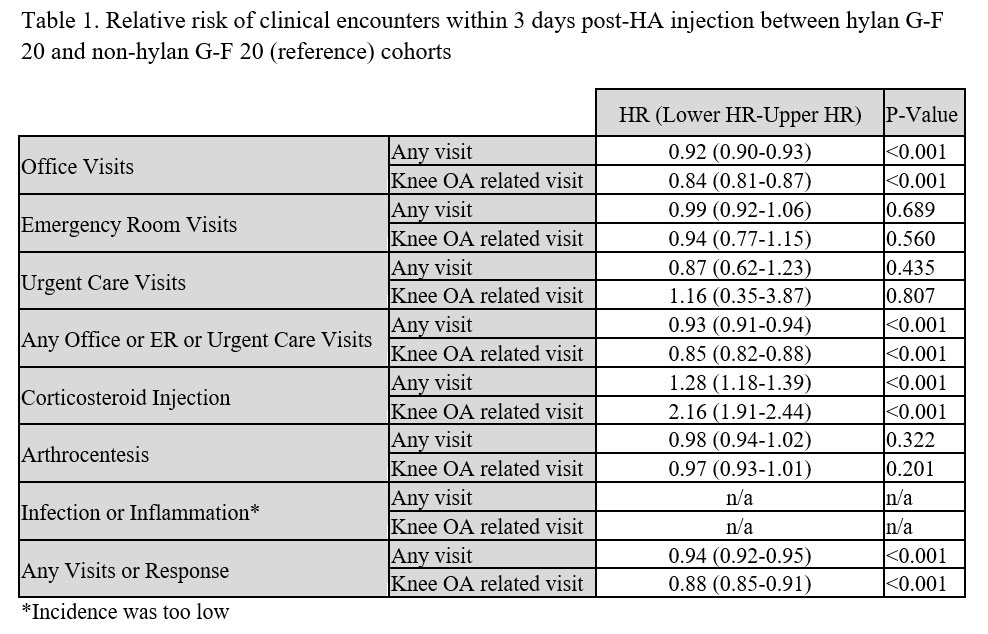
Results: The study cohort involved 748,428 HA patients with 23.2% in the hylan G-F 20 group. Knee OA-related inflammation/infection rate was 0.001% for hylan G-F 20 and 0.002% for non-hylan G-F 20 groups (Fig. 1). Knee OA-related arthrocentesis/aspiration rate was 1.6% for hylan G-F 20 and 2.1% for non-hylan G-F 20 groups, with comparable risk for both groups (p=0.201; Table 1). The rate of CS injection (any diagnosis) was 0.48% for hylan G-F 20 patients compared to 0.41% for non-hylan G-F 20 patients, with 28% greater risk greater for hylan G-F 20 patients (p< 0.001), but the combined rates of CS injection and arthrocentesis/aspiration (any diagnosis) was comparable for both groups (hylan G-F 20: 2.2%; non-hylan G-F 20: 2.6%). The risk of any visit or studied responses was lower for the hylan G-F 20 cohort by 12% (unadjusted rate of 3.1% versus 4.3%; p< 0.001). Clinical characteristics, such as CS injections or arthroscopy within 1 week before HA and ultrasound imaging, were associated with 84%, 289%, and 35% increased risk of any studied clinical encounter, respectively. Concomitant CS and HA injections, and fluoroscopic imaging were associated with 35% and 42% lower risks, respectively (all p< 0.001).
Conclusion: Our study of almost 750,000 knee OA patients who had HA injections, of which about a quarter were only given hylan G-F 20, demonstrated that the diagnosis of inflammations or infections within three days of the HA injection was extremely rare. The occurrence was 1 out of 100,000 hylan G-F 20 patients and 2 out of 100,000 non-hylan G-F 20 patients for those events that had a corresponding knee OA diagnosis, and increased to 2 out of 10,000 for both cohorts when all inflammation or infections were included regardless of diagnosis. The overall risk of surrogate SALR measures was not greater for hylan G-F 20 patients.
To cite this abstract in AMA style:
Ong K, Runa M, Ngai W, Xiao Z, Lau E, Altman R. Severe Acute Localized Reactions Following Intra-Articular Hyaluronic Acid Injections in Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/severe-acute-localized-reactions-following-intra-articular-hyaluronic-acid-injections-in-knee-osteoarthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/severe-acute-localized-reactions-following-intra-articular-hyaluronic-acid-injections-in-knee-osteoarthritis/