Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis genetic risk scores (RA-GRS) improve RA risk prediction, but the added predictive value over lifestyle risk factors is modest. Several human leukocyte antigen (HLA) haplotypes are strongly related to seropositive RA. Recently, we identified a number of metabolites, including short-chain acylcarnitines and metabolites involved in polyamine metabolism, associated with RA risk. Integrating RA-GRS and metabolomics may provide insight into RA pathogenesis.
Methods: Plasma samples obtained prior to disease onset from 254 female participants who later developed validated RA in the Nurses’ Health Studies were 1) genotyped, imputed to the 1000 Genomes reference panel, and imputed HLA haplotype using SNP2HLA, and 2) analyzed using untargeted liquid-chromatography tandem mass-spectrometry for metabolomic profiling. Genetic risk measures included: 93 weighted non-HLA single nucleotide variants (wGRS93), and 18 weighted HLA (wHLA) haplotypes previously associated with RA risk. For metabolomic measurements, three complementary methods were applied to detect amines and polar metabolites, polar and nonpolar lipids, and free fatty acids, bile acids, and metabolites of intermediate polarity. After quality control, 360 unique metabolites were included for analysis. Associations between wGRS93, wHLA, and the cumulative sum of wGRS93 and wHLA and individual metabolite levels were tested using ordinary least squares to identify putative genetically informed metabolites of RA. Adjustment for multiple comparisons was made using the pooled local index of significance, a false discovery rate approach which employs Hidden Markov Models to account for dependency structures in the data.
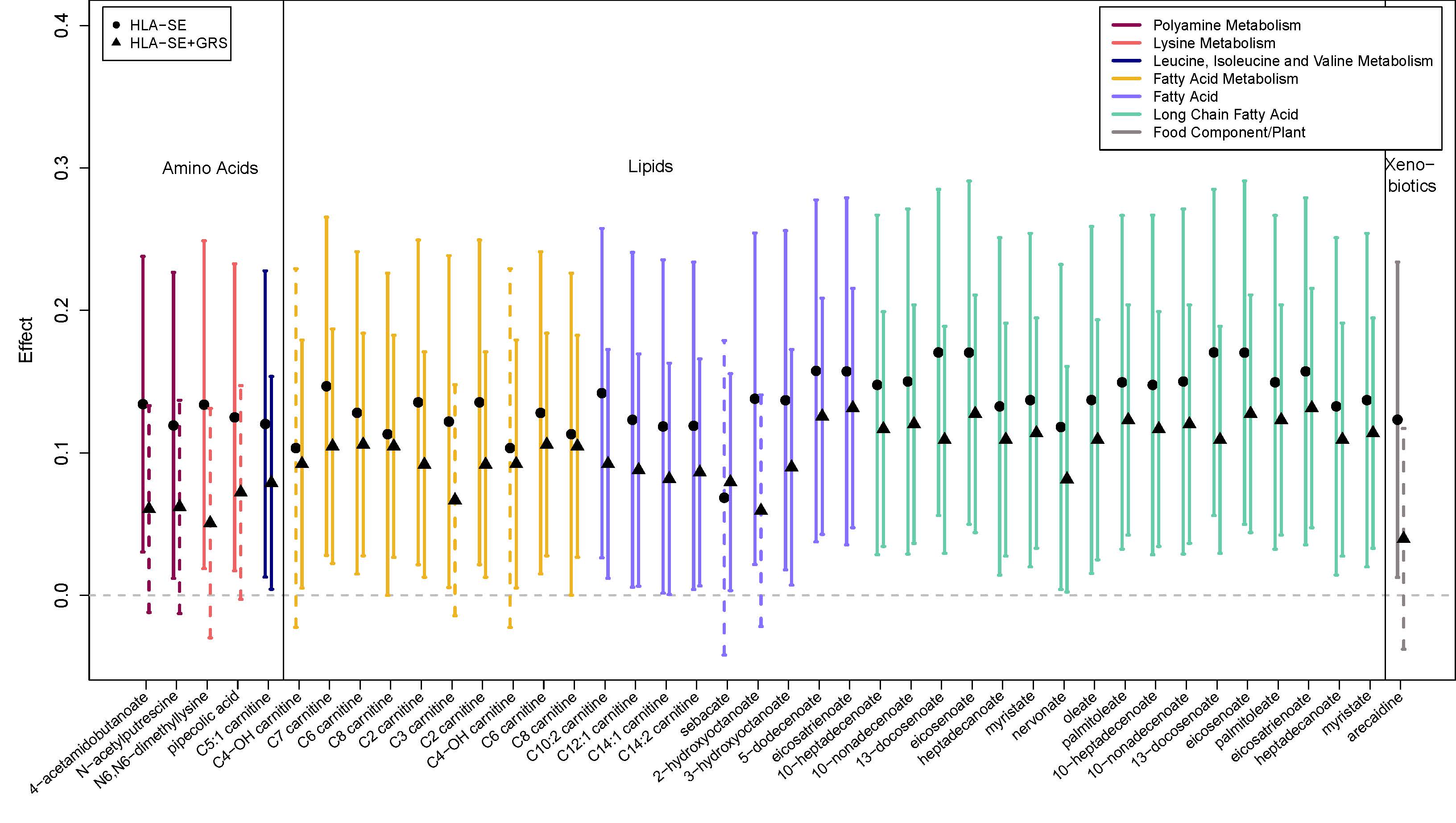
Results: After adjustment for multiple comparisons, no genetically informed metabolites for wGRS93 were found; however, 27 RA wHLA-SE associations were identified, including several short-chain acylcarnitines and metabolites involved in polyamine metabolism that we previously found to be associated with RA: C2 carnitine (β=0.120; p=0.018), C3 carnitine (β=0.122; p=0.007), C5:1 carnitine (β=0.120; p=0.016), 4-acetamidobutanoate (β=0.134; p=0.027), and N-acetylputrescine (β=0.119; p=0.011). In the combined wGRS93 and wHLA-GRS score, 21 associations were identified, also including several carnitines identified for wHLA-SE, as well as additional acylcarnitines: C4-OH carnitine (β=0.092; p=0.048) and C7 carnitine (β=0.105; p=0.017). In all results, higher genetic risk was associated with increased standardized metabolite expression levels.
Conclusion: We identified several novel associations between both 1) wHLA haplotypes and 2) combined wGRS93 and wHLA risk scores, with newly identified metabolite levels in pre-RA, but none for GRS93. These associations may point to new biologic pathways involved in RA development. However, further validation is required.

Chu-ACR2019-NHSRA-PRSxMetab-Table1
To cite this abstract in AMA style:
Chu S, Cui J, Sparks J, Lu B, Clish C, Lasky-Su J, Karlson E, Costenbader K. Integrating Genetic Risk Scores and Pre-Diagnostic Metabolomics to Infer Dysregulated Mechanisms in Rheumatoid Arthritis in Women [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/integrating-genetic-risk-scores-and-pre-diagnostic-metabolomics-to-infer-dysregulated-mechanisms-in-rheumatoid-arthritis-in-women/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrating-genetic-risk-scores-and-pre-diagnostic-metabolomics-to-infer-dysregulated-mechanisms-in-rheumatoid-arthritis-in-women/