Session Information
Date: Monday, November 11, 2019
Title: 4M119: Sjögrenʼs Syndrome – Basic & Clinical Science (1902–1907)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Chemokine (C-X-C motif) ligand 13 (CXCL13), produced by follicular helper T (Tfh) cells, plays a pivotal role in B-cell homing and activation in germinal centers in salivary glands.1,2 CXCL13 is associated with systemic disease activity and clinical activity in primary Sjögren’s syndrome (pSS) and increased risk of lymphoma.1,2 In patients with pSS, in an open-label trial (ASAP), abatacept (ABA) treatment decreased Tfh and CD4 memory T (TEM) cells.1 In the Phase III, double-blind, placebo-controlled study (NCT02915159), ABA significantly impacted biomarkers of disease activity, but did not improve clinical disease measures, vs placebo.3 This analysis aimed to assess serum CXCL13 levels and immune cell phenotypes of study patients.
Methods: Adult patients meeting the 2016 ACR/EULAR criteria for pSS with EULAR Sjögren’s Syndrome Disease Activity Index ≥5 were randomized to receive ABA 125 mg SC weekly or placebo for 168 days. Serum and whole blood samples were collected at follow-up. Serum CXCL13 was measured using the SIMOA™ assay from Myriad RBM (all patients). Immune cell phenotyping of whole blood samples in a subgroup of patients from participating countries with available samples (cellular phenotyping population [CPP]) was assessed by flow cytometry, with data analyzed using BD FACSDiva™ software. Estimates of adjusted mean change from baseline (BL) in immune cells were from a repeated measures mixed model with statistical significance determined by the Benjamini–Hochberg procedure.
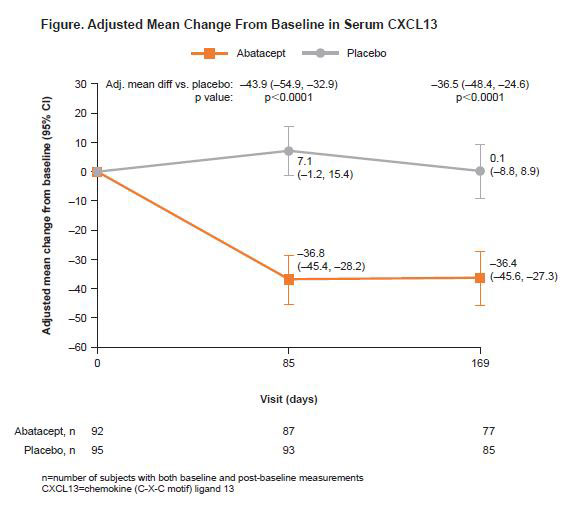
Results: In total, 187 (ABA, 92; placebo, 95)3 patients were randomized and received ≥1 dose of study drug. At BL, CXCL13 levels were comparable between treatment arms. At Days 85 and 169, ABA reduced levels of serum CXCL3 from BL, and ABA-treated patients had significantly lower levels of serum CXCL13, compared with placebo (p< 0.0001; Figure). Seventy-eight (ABA, 32; placebo, 46) patients were included in the CPP, with no observed differences in BL characteristics between treatment groups or populations (CPP vs non-CPP). ABA-treated patients had significantly reduced proportions of disease-relevant cell subsets (Tfh, activated Tfh, CD4 TEM, helper T cell 1 [Th1] and regulatory T cells [Treg]) at Day 169 compared with BL (data not shown). At Day 169, there was a significant adjusted mean difference in adjusted mean change from BL for ABA vs placebo for Tfh, ICOS-expressing activated Tfh and Treg cells (Table), and CD4 TEM, Th1 cells and naïve CD4 T cells (data not shown), and a numerical difference for plasmablasts (Table).
Conclusion: A significant pharmacodynamic effect of abatacept was observed on disease-relevant serum (CXCL13) and pathogenic cell subpopulations. The disconnect between the effectiveness of abatacept in lowering serum CXCL13 (a relevant biomarker in pSS) and pathogenic cell subsets, and the previously-reported lack of demonstrable benefit (vs placebo) in clinical outcomes, warrants further investigation.
1Verstappen GM, et al. Arthritis Rheumatol 2017;69:1850–61.
2Nocturne G, et al. Arthritis Rheumatol 2015;67:3226–33.
3Baer AN, et al. EULAR 2019:abstract OP0039.
Professional medical writing: Rachel Rankin, Caudex, funded by Bristol-Myers Squibb.
To cite this abstract in AMA style:
Gottenberg J, Mukherjee S, Nys M, Wong R, Bootsma H, Ray N. Abatacept Reduces Serum CXCL13 and Disease-Relevant Immune Cell Phenotypes in a Double-Blind, Placebo-Controlled Primary Sjögren’s Syndrome Clinical Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/abatacept-reduces-serum-cxcl13-and-disease-relevant-immune-cell-phenotypes-in-a-double-blind-placebo-controlled-primary-sjogrens-syndrome-clinical-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/abatacept-reduces-serum-cxcl13-and-disease-relevant-immune-cell-phenotypes-in-a-double-blind-placebo-controlled-primary-sjogrens-syndrome-clinical-trial/