Session Information
Date: Monday, November 11, 2019
Title: 4M118: Reproductive Issues in Rheumatic Disorders (1896–1901)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Pregnancy counseling of all anti-Ro positive women includes advice regarding the development of congenital heart block (CHB), albeit the risk is only 2% for primigravida women or those with previously unaffected offspring. Despite this low risk, the prevailing surveillance recommendation is weekly echocardiography. While evidence from basic research laboratories support that “high” titers of antibodies confer clinically meaningful risk, unfortunately the majority of commercial laboratories use the BioPlex assay, which provides positive and negative values with limited information on actual levels because the sera or plasma are not diluted past a specified cutoff given cost (e.g. values of anti-Ro inclusive of Ro52 or Ro60 by laboratories such as Quest or LabCorp provide positive as 1-8 or > 8 units with no further information). The present study was initiated to assess whether the BioPlex assay used by many commercial laboratories provides adequate stratification of risk for counseling regarding management.
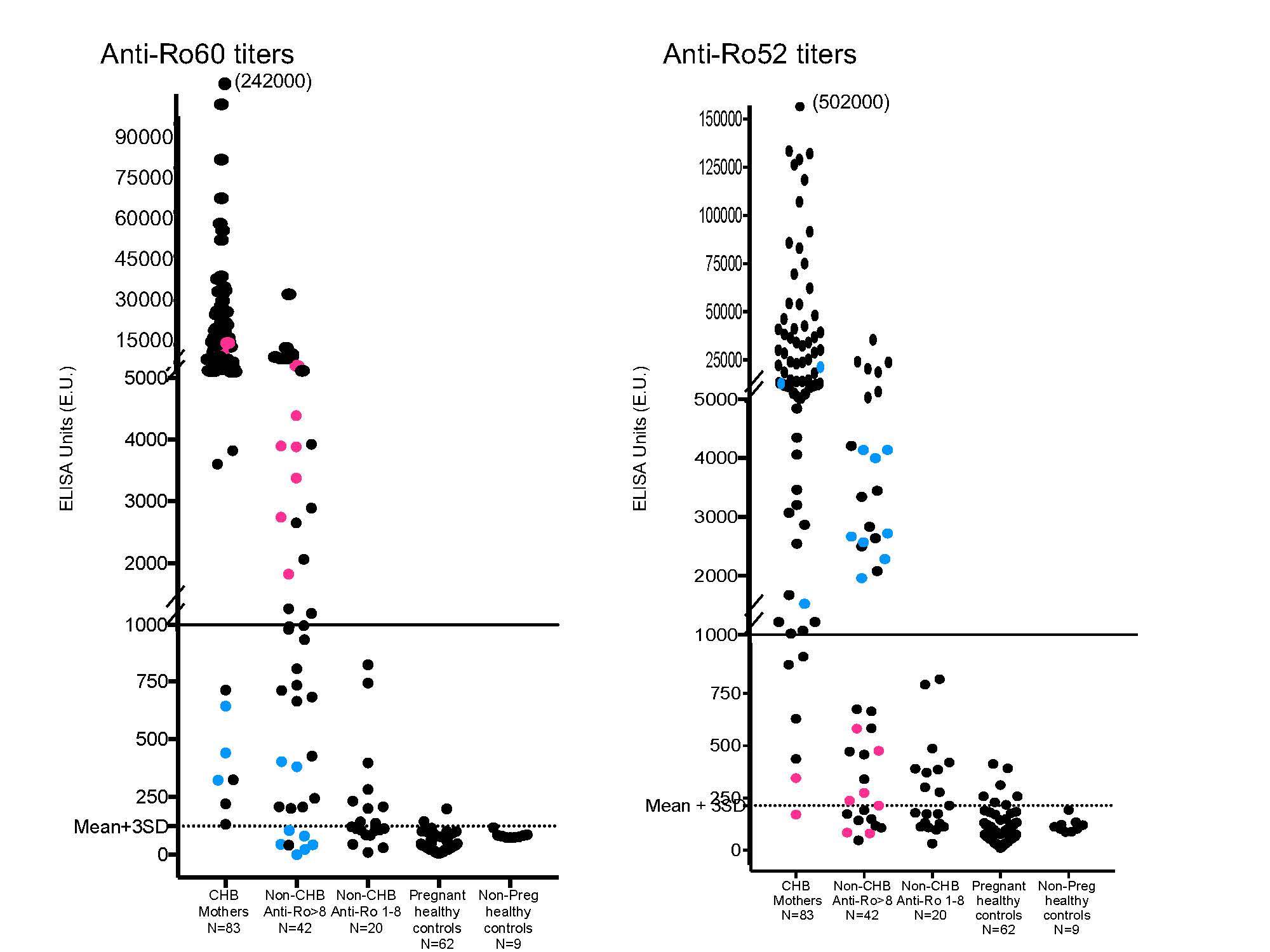
Methods: The study group comprised healthy non-pregnant donors (N = 9), healthy pregnant donors (N = 62), women testing positive for anti-Ro by commercial BioPlex but without CHB children (N = 60 SLE and 2 SS), and women with CHB children (N = 83). Anti-Ro60 reactivity was assessed using native antigen and anti-Ro52 using recombinant protein. Sera were applied to coated microtiter plates at serial dilutions ranging from 1:1000 – 1:50,000 for 1h at RT and run in duplicate. Tested samples were multiplied by the dilution factor which gives an OD in the range of 0.3-0.8. Results were considered positive at 123 ELISA units (EU) for Ro60 and 215 EU for Ro52 as this represented the mean +3 SD of the values obtained for healthy control sera.
Results: Of the 83 CHB mothers tested, 74 had titers of Ro60 and Ro52 > 1000 EU, in 1 anti-Ro60 was > 1000 EU and anti-52 Ro between 215 – 1000, in 3 anti-Ro52 was > 1000 EU and anti-Ro60 between 300 – 1000, and 1 mother had anti-Ro60 > 1000 EU and was negative for anti-Ro52. Albeit all positive, the sera from 4 CHB mothers obtained 15 years after the birth of the affected child were < 1000 EU for both anti-Ro60 and Ro52. With these results setting thresholds ( > 1000 EU in either Ro60 or Ro52 for CHB risk), we assessed patients testing positive for anti-Ro based on the BioPlex assay. Of 42 patients with values of > 8 on BioPlex testing, 14 had titers > 1000 EU for both anti-Ro60 and Ro52, 7 had anti-Ro60 > 1000 EU, and 8 had anti-Ro52 > 1000 EU. Thus, 13 of 42 (25%) with commercial Ro > 8 did not meet the threshold EU for CHB risk. Of 20 patients considered positive for anti-Ro by BioPlex with values between 1-8, none had levels of either anti-Ro60 or Ro52 at 1000 EU. No patient or healthy control testing negative by the BioPlex assay was positive for CHB risk in our ELISA.
Conclusion: These data suggest that commercial testing using the BioPlex assay may fall short of stratifying risk for CHB. Women with positive values < 8 are not likely at risk, obviating the cost and burden of weekly fetal echo surveillance. Moreover, even those considered “high” titer on commercial testing may be at low risk supporting the need for more quantitative commercial testing than is currently available.
To cite this abstract in AMA style:
Robins K, Bhan R, Trad C, Cohen R, Chang M, Wainwright B, Masson M, Mehta-Lee S, Izmirly P, Clancy R, Cuneo B, Buyon J. Assessing Commercial Titers of anti-Ro60 and Ro52 Antibodies to Risk Stratify Surveillance of Anti-Ro/SSA Antibody Positive Pregnancies [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/assessing-commercial-titers-of-anti-ro60-and-ro52-antibodies-to-risk-stratify-surveillance-of-anti-ro-ssa-antibody-positive-pregnancies/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-commercial-titers-of-anti-ro60-and-ro52-antibodies-to-risk-stratify-surveillance-of-anti-ro-ssa-antibody-positive-pregnancies/