Session Information
Date: Monday, November 11, 2019
Title: 4M097: Systemic Sclerosis & Related Disorder – Clinical II: Cardiopulmonary Involvement (1830–1835)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Observational studies have demonstrated that African American (AA) patients with systemic sclerosis (SSc) have a more unfavorable prognosis compared with non-AA. However, no studies have evaluated racial disparities using data from a randomized controlled trial (RCT) where all patients have equal access to care and standard treatment and follow-up during the trial. The objective of this study was to compare efficacy outcomes, as well as long-term morbidity and mortality, in AA and non-AA patients who participated in the Scleroderma Lung Study (SLS) I (Tashkin et al. NEJM 2006) and II (Tashkin et al. Lancet Resp Med 2016).
Methods: SLS I randomized 158 SSc participants (AA: N=26) with interstitial lung disease (ILD) from 13 US SSc centers to 1 year of oral CYC (cyclophosphamide) versus placebo, followed by 1 year off all treatment. SLS II randomized 142 SSc-ILD participants (AA: N=33) from 14 US SSc centers to 1 year of oral CYC, followed by 1 year of placebo, versus 2 years of mycophenolate (MMF). Both studies measured the forced vital capacity (FVC), diffusing capacity for carbon monoxide (DLCO) every 3 months during the study period. Up to 12 (SLS I) and 8 (SLS II) years after randomization, we contacted enrolled patients or designated surrogates to assess the following: mortality, cause of mortality, and development of organ failure. We created joint models to compare progression of ILD based on the course of the FVC and DLCO between AA and non-AA. We compared survival rates using a log-rank test and used Cox proportional hazard modeling to determine the variables associated with survival.
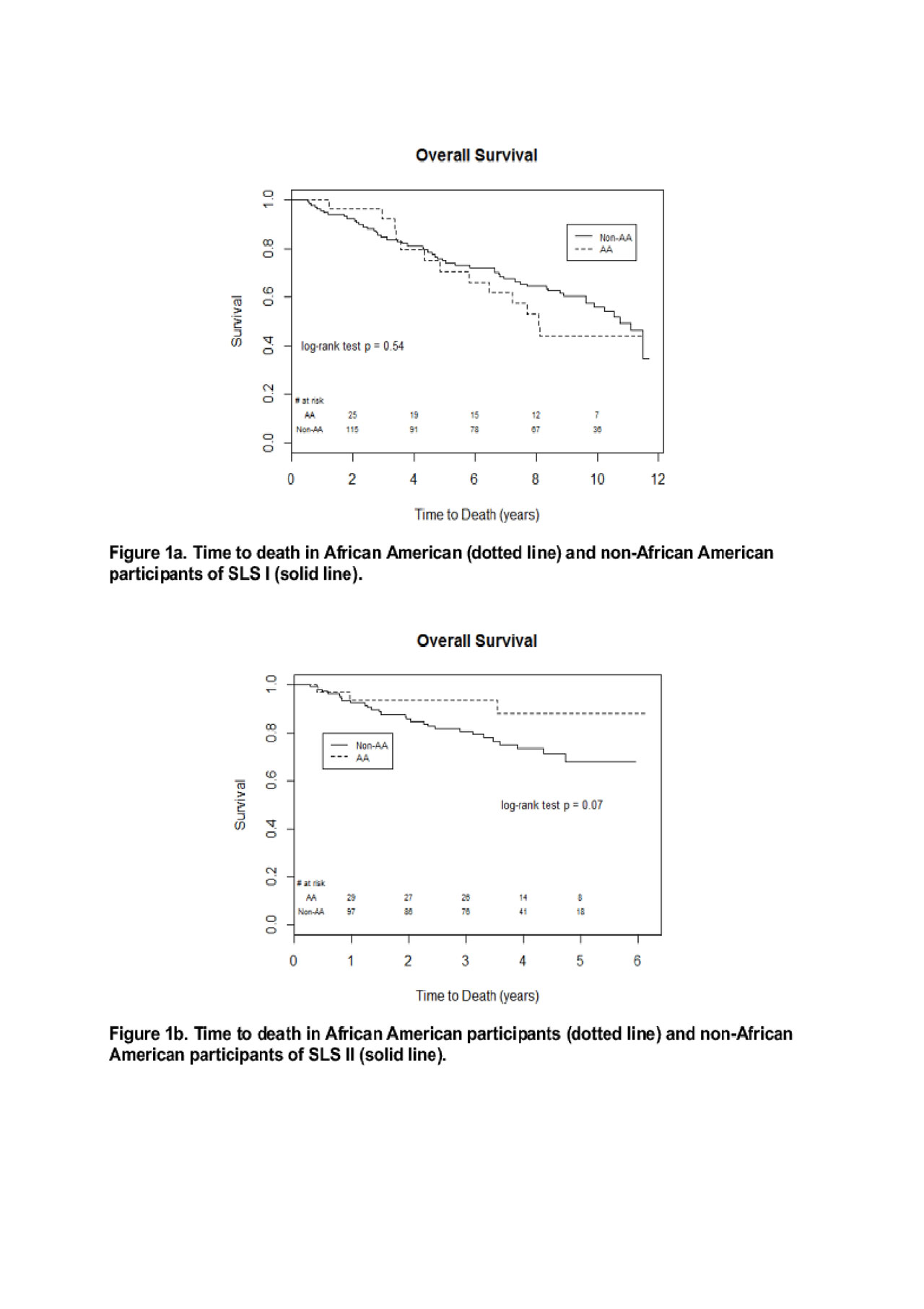
Results: Baseline demographic and disease characteristics of AA and non-AA were similar in SLS I and II, with the exception of age (AA were younger than non-AA [SLS I: 43.1 vs. 49.5 years; SLS II: 49.1 vs. 53.2 years], and extent of ILD (AA had a trend for more extensive quantitative radiographic ILD at baseline). In SLS I, there was no significant difference in the course of the FVC or DLCO over 24 months between AA and non-AA in CYC or placebo arms. In SLS II, AA had an improved course of FVC over 24 months compared with non-AA in the CYC arm (P=0.02); in the MMF arm, there was no difference in the course of the FVC. There was also no difference in the course of the DLCO over 24 months in either CYC or MMF arms. There was no difference in long-term survival (P=0.54; Figure 1a) or time to respiratory failure (P=0.37) between AA and non-AA in SLS I. There was a trend for improved long-term survival P=0.07; Figure 1b) and improved time to respiratory failure (P=0.10) in AA compared with non-AA in SLS II. The Cox models demonstrated AA race did not have any significant impact on long-term survival in SLS I or II participants.
Conclusion: Data from two large RCTs in SSc-ILD demonstrated that AA patients with SSc-ILD have similar morbidity and mortality outcomes compared with non-AA SSc-ILD patients when treated aggressively for SSc-ILD, even after adjusting for age and baseline disease severity. These findings contrast with the racial disparities described in previous observational studies and warrant further investigation.
To cite this abstract in AMA style:
Volkmann E, Steen V, Li N, Roth M, Clements P, Khanna D, Furst D, Assassi S, Kim G, Goldin J, Elashoff R, Tashkin D. Short- and Long-term Morbidity and Mortality Outcomes of African American Patients with Systemic Sclerosis-Related Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/short-and-long-term-morbidity-and-mortality-outcomes-of-african-american-patients-with-systemic-sclerosis-related-interstitial-lung-disease/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-and-long-term-morbidity-and-mortality-outcomes-of-african-american-patients-with-systemic-sclerosis-related-interstitial-lung-disease/