Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: TNF-alpha inhibiting therapies (TNFi) are a cornerstone of treatment for inflammatory conditions such as IBD, JIA, and chronic noninfectious osteomyelitis (CNO) but have been increasingly implicated in the development of psoriasis. The objective of this study was to compare the rate of psoriasis in children with IBD, JIA, and CNO with TNFi exposure to those without TNFi exposure and to the general pediatric population.
Methods: This was a single-center retrospective cohort study of electronic health record data from 2008 to 2018. Inclusion criteria were at least 2 ICD-9 or ICD-10 codes for JIA, IBD, or CNO, age at diagnosis of under 19 years, and at least 2 visits with a study center rheumatologist or gastroenterologist due to the frequent nature of one-time second opinion visits. Subjects with a listed past medical history or diagnosis code of psoriasis prior to the diagnosis of JIA, IBD, or CNO were excluded. TNFi exposure was defined as at least 1 prescription for either adalimumab, etanercept, or infliximab, and subjects were dichotomized as “ever” or “never” having TNFi exposure. The primary outcome was incident psoriasis which was defined as the first ICD-9 or ICD-10 code for psoriasis during a visit with a study center rheumatologist, gastroenterologist, or dermatologist. Subjects who had not developed psoriasis by their final recorded visit were considered censored. Incidence rates (IRs) were calculated as well as standardized incidence ratios (SIRs) comparing the observed to expected number of psoriasis cases in the general pediatric population according to previously published data. Univariate and multivariate Cox proportional hazards models determined by stepwise model selection were used to estimate the strength of association between TNFi exposure and incident psoriasis and to evaluate risk factors associated with psoriasis development.
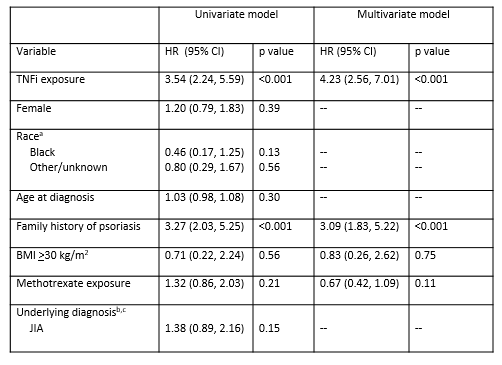
Results: Between January 2008 and October 2018, 4403 children met inclusion criteria. Of these children, 1738 (39%) had TNFi exposure and 2665 (61%) did not with 5245 and 7365 person-years of follow-up, respectively. The IRs and SIRs for the entire cohort and for each condition are presented in Table 1. There were 64 (IR 1.2 per 100 person-years) and 26 (IR 0.4 per 100 person-years) cases of psoriasis in children with and without TNFi exposure, respectively. The SIR was 17.5 (95% CI 14.2, 21.5) for the entire cohort, 29.9 (95% CI 23.4, 38.2) for the TNFi exposed group, and 8.7 (95% CI 5.9, 12.7) for the TNFi unexposed group. When holding all other covariates constant, the hazard ratio for psoriasis comparing TNFi exposure to no TNFi exposure was 4.23 (95% CI 2.56, 7.01, p< 0.001) (Table 2). In children with TNFi exposure, family history of psoriasis was independently associated with an increased hazard of psoriasis (HR 3.09, 95% CI 1.83, 5.22, p< 0.001) (Table 2).
Conclusion: Children with IBD, JIA, and CNO had an increased rate of psoriasis compared to the general pediatric population, with the highest rate in those with TNFi exposure. TNFi treatment in children with IBD and JIA was associated with an increased hazard of developing psoriasis compared to those not treated with TNFi.
aSIRs were calculated as the ratio of observed psoriasis cases to expected psoriasis cases as estimated by the incidence in the general pediatrics population -40.8 per 100,000- -Tollefson, et al. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010; 62-6-: 979-87-. A SIR >1 indicates that more cases were observed than expected.
BMI: body mass index; HR: hazard ratio; CI: confidence interval
aReference is white; bReference is IBD; cCNO removed from both models
due to small sample size and low event rate.
To cite this abstract in AMA style:
Buckley L, Xiao R, Perman M, Grossman A, Weiss P. Psoriasis Associated with Anti-Tumor Necrosis Factor-Alpha Therapies in Children with Inflammatory Bowel Disease, Juvenile Idiopathic Arthritis, and Chronic Noninfectious Osteomyelitis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/psoriasis-associated-with-anti-tumor-necrosis-factor-alpha-therapies-in-children-with-inflammatory-bowel-disease-juvenile-idiopathic-arthritis-and-chronic-noninfectious-osteomyelitis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/psoriasis-associated-with-anti-tumor-necrosis-factor-alpha-therapies-in-children-with-inflammatory-bowel-disease-juvenile-idiopathic-arthritis-and-chronic-noninfectious-osteomyelitis/