Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: SSc-ILD is a major cause of morbidity and mortality in patients with systemic sclerosis. A subset of patients with SSc-ILD show a decline in lung function and increasing symptoms that interfere with daily activities and ultimately impair patients’ health-related quality of life (HRQoL). The aim of this analysis was to evaluate the impact of lung function changes on HRQoL as measured by patient-reported outcomes (PRO) within the Phase III SENSCIS® trial.
Methods: In the SENSCIS® trial (NCT02597933), patients with SSc-ILD were randomized to nintedanib 150 mg twice daily or placebo. Patients in the nintedanib arm experienced a significantly lower adjusted annual rate of decline in forced vital capacity (FVC) (–52.4mL vs –93.3mL: difference 41.0 [95% CI 2.9–79.0]; P=0.04). PRO assessments were conducted at baseline, week 24, week 52 and study completion, with no differences between arms in changes from baseline to week 52 in the St. George’s Respiratory Questionnaire (SGRQ). In this analysis, PRO scores and changes from baseline were assessed according to baseline lung function parameters, independent of treatment arm. PRO assessments included Functional Assessment of Chronic Illness Therapy (FACIT)-Dyspnea, Health Assessment Questionnaire Disability Index (HAQ-DI) and Scleroderma Health Assessment Questionnaire (SHAQ) visual analog scale (VAS).
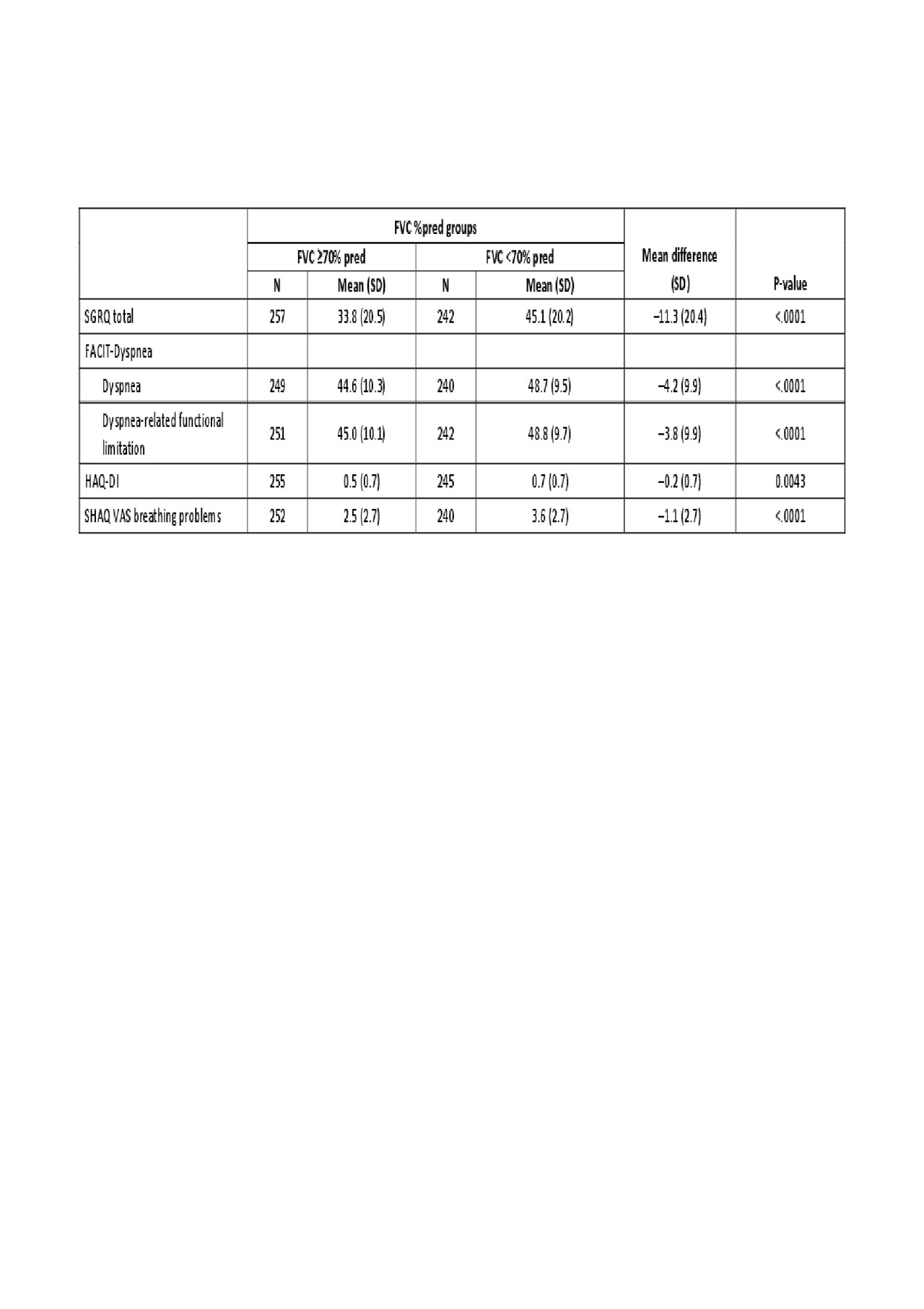
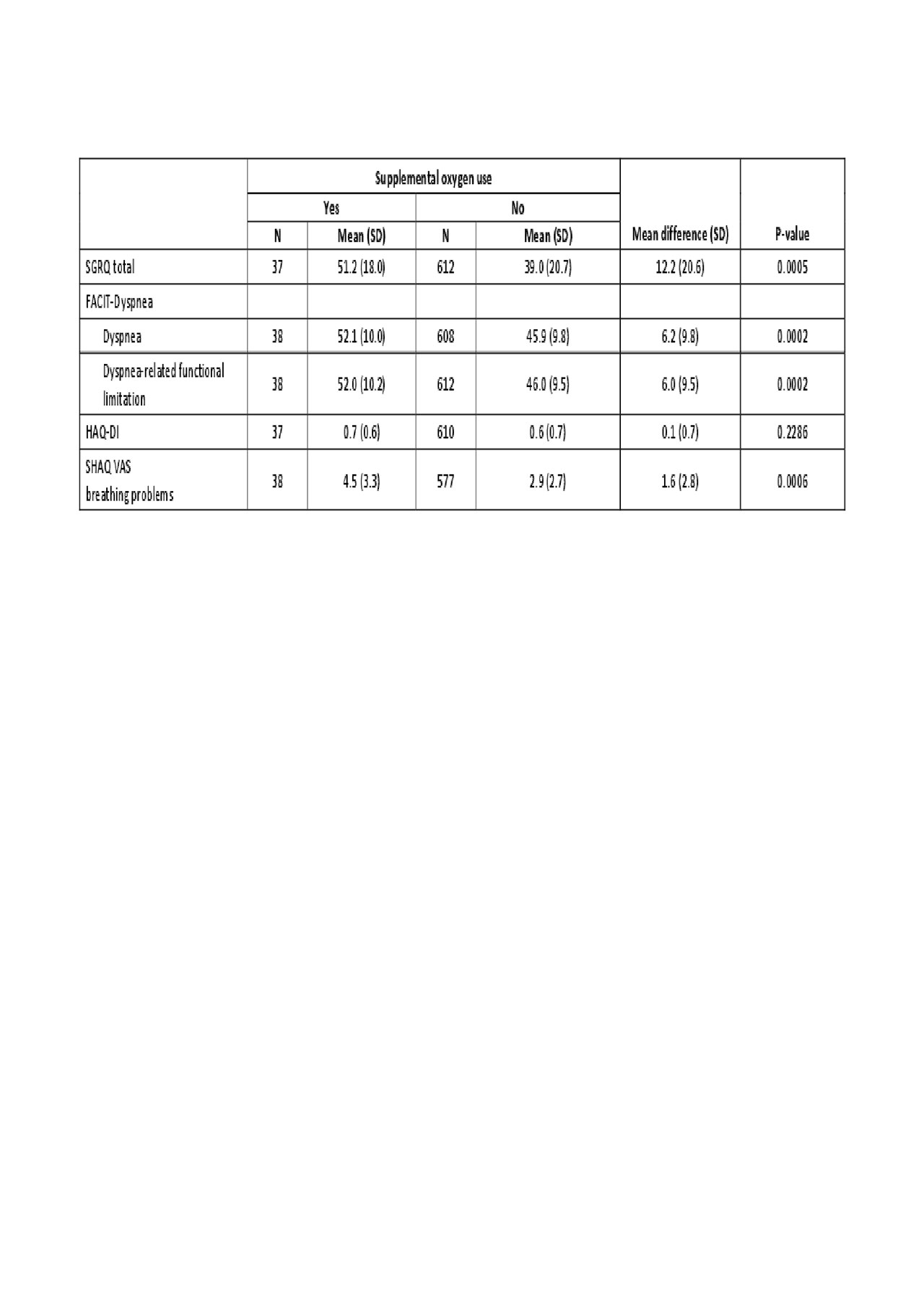
Results: A total of 576 patients were randomized. In patients categorized by baseline FVC percent predicted (FVC %pred), those with FVC ≥70% pred had a mean SGRQ total score of 33.8 at week 52, whereas mean score for those with FVC < 70% pred was 45.1 (P< 0.0001) (higher scores indicate a worse quality of life). This pattern was also reflected for the subdomains of the SGRQ score, including activities, impacts and symptoms. Similarly, FACIT-Dyspnea, FACIT-Dyspnea functional limitations, HAQ-DI and SHAQ VAS breathing problems scores were lower in patients with FVC ≥70% pred than those with FVC < 70% pred (Table 1). When PRO assessments were compared in patients categorized by supplemental oxygen use at baseline, those using oxygen had a mean SGRQ total score of 51.2, compared with 39.0 in those not requiring oxygen (P=0.0005). Again, these differences were also evident for the subdomains of the SGRQ score. FACIT-Dyspnea, HAQ-DI and SHAQ VAS breathing problems scores were also all higher in patients with oxygen use (Table 2). In patients with a moderate (5–10%) or large ( >10%) improvement in FVC %pred from baseline to week 52, changes in SGRQ scores indicated improved HRQoL (–3.4 and –3.8, respectively). A moderate or large deterioration in FVC %pred was associated with worsening HRQoL (+1.4 and +5.5, respectively). Other PRO assessments showed a largely similar pattern (Table 3).
Conclusion: In the SENSCIS® trial, while there was no significant difference between arms in the change from baseline in SGRQ, patients with FVC < 70% pred or requiring supplemental oxygen had consistently worse PRO scores, independent of treatment arm. Improvements in lung function between baseline and week 52 were reflected in improving PRO scores, potentially indicating an association between lung function and HRQoL in patients with SSc-ILD.
To cite this abstract in AMA style:
Kreuter M, Hoffmann-Vold A, Matucci-Cerinic M, Saketkoo L, Highland K, Wilson H, Alves M, Schoof N, Maher T. Health-Related Quality of Life in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD): Impact of Lung Function on Patient-Reported Outcomes in a Randomized Phase III Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/health-related-quality-of-life-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-impact-of-lung-function-on-patient-reported-outcomes-in-a-randomized-phase-iii-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/health-related-quality-of-life-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-impact-of-lung-function-on-patient-reported-outcomes-in-a-randomized-phase-iii-trial/