Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Ixekizumab (IXE), a high affinity monoclonal antibody that selectively targets interleukin-17A, has been demonstrated to improve the signs and symptoms, disease activity, and quality of life of patients with active PsA. SPIRIT-P3 was a randomized withdrawal (RW) phase 3b clinical trial that demonstrated that in patients who achieved sustained minimal disease activity (MDA) after open label (OL) IXE treatment, continued IXE treatment was superior to IXE withdrawal (placebo, PBO) in maintaining MDA. In this pre-specified and post-hoc analysis, we assessed the impact of IXE withdrawal on the individual components of MDA.
Methods: In SPIRIT-P3 (NCT02584855), patients with active PsA who were naïve to biologic DMARDs received OL treatment with IXE 80 mg every two weeks (Q2W) after a 160-mg starting dose for at least 36 weeks. Patients who achieved MDA (at least 5 out of 7 components [see table]) for three consecutive months between Weeks 36 and 64 were flexibly randomized 1:1 to IXE Q2W or placebo (PBO) and evaluated up to Week 104 in a double-blind manner. Patients were considered relapsed if they lost MDA at any point during the RW and received IXEQ2W thereafter. Kaplan-Meier product limit method was used to estimate time to loss of response of individual components in the RW intent-to-treat population. Treatment comparisons were done between groups using adjusted log-rank test stratified by geographic region and conventional synthetic DMARDs (cDMARD) use. A p-value < 0.05 was considered to be significant. Number of components of MDA met at randomization and at time of relapse in both PBO and IXE groups were assessed in the relapse population.
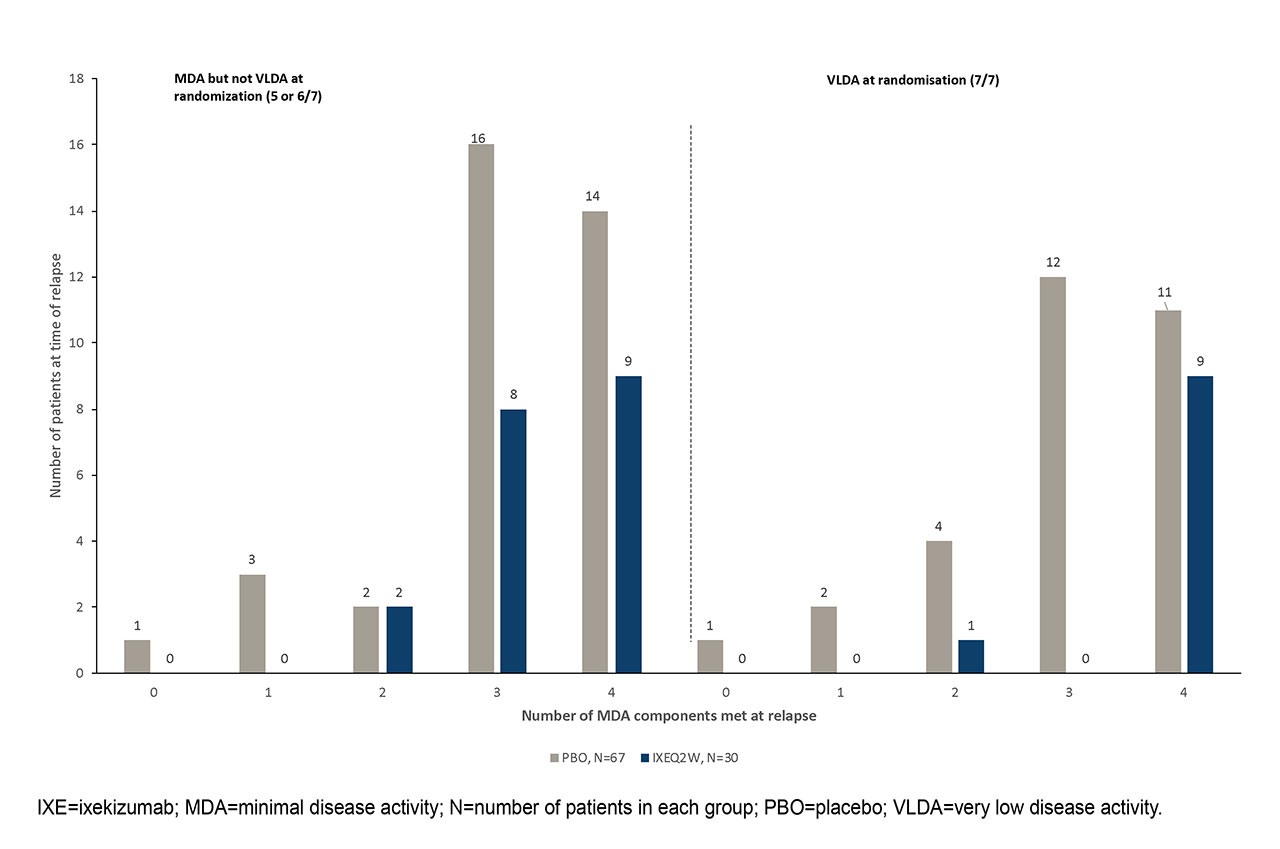
Results: A total of 394 patients entered into the OL treatment period; 158 (40%) of whom met three consecutive months of MDA and randomized to IXE (N=79) or PBO (N=79) in the RW period. At time of randomization, 77 patients (37 assigned to PBO; 40 assigned to IXEQ2W) had achieved very low disease activity (VLDA; i.e., meeting 7 out of 7 components). Overall, significantly more patients randomized to PBO than IXE relapsed during the RW period (data not shown). Among patients who had VLDA at randomization, 30 (81%) of PBO patients and 10 (25%) of IXE patients met relapse criteria. Time to relapse was significantly shorter in patients on PBO than IXE-treated patients for 5 out of 7 individual components (Tender joint count, Swollen joint count, PASI/BSA, patient pain VAS, Patient Global Disease Activity VAS) of MDA (p < 0.01, Table). Among patients who relapsed, 18 (60%) from IXEQ2W and 25 (37%) from PBO groups met 4 out of 7 components at time of relapse (figure).
Conclusion: In patients with sustained MDA, IXE withdrawal led to relapse in components of MDA, particularly tender and swollen joints, patient reported joint pain and disease activity, and psoriasis. IXE withdrawal, relative to continued treatment, was associated with relapse in multiple components of MDA within individual patients, even in patients who had achieved VLDA.
To cite this abstract in AMA style:
Coates L, Pillai S, Hufford M, Alves D, Young Park S, Gallo G, Wu B, Valter I, Tahir H, Chandran V, Kavanaugh A, Mease P. Withdrawal of Ixekizumab Results in Loss of Efficacy in Multiple Clinical Domains in Patients with Psoriatic Arthritis Who Had Achieved Minimal Disease Activity: Results from the SPIRIT-P3 Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/withdrawal-of-ixekizumab-results-in-loss-of-efficacy-in-multiple-clinical-domains-in-patients-with-psoriatic-arthritis-who-had-achieved-minimal-disease-activity-results-from-the-spirit-p3-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/withdrawal-of-ixekizumab-results-in-loss-of-efficacy-in-multiple-clinical-domains-in-patients-with-psoriatic-arthritis-who-had-achieved-minimal-disease-activity-results-from-the-spirit-p3-study/