Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Multiple treatment options are available for patients with RA. ACR guidelines recommend initiating treatment with a conventional synthetic DMARD (csDMARD). If a patient fails to achieve the treat-to-target goal, ACR guidelines recommend adjusting therapy by adding another csDMARD or switching to another mechanism of action. The aim of this study was to evaluate patient characteristics and treatment patterns and persistency in RA patients who have previously been treated with a biologic DMARD (bDMARD).
Methods: In the Corrona US RA Registry, we identified instances when adult bDMARD-experienced patients initiated another treatment regimen (index date, between 01/2013–11/2018) and had a 6-month follow-up visit. New regimens were categorized as csDMARDs, tumor necrosis factor inhibitors (TNFi), and non-TNFi bDMARDs or Janus kinase inhibitors (JAKi). Fisher’s exact test was used for categorical variables and Kruskal-Wallis or Wilcoxon rank-sum tests were used for continuous variables.
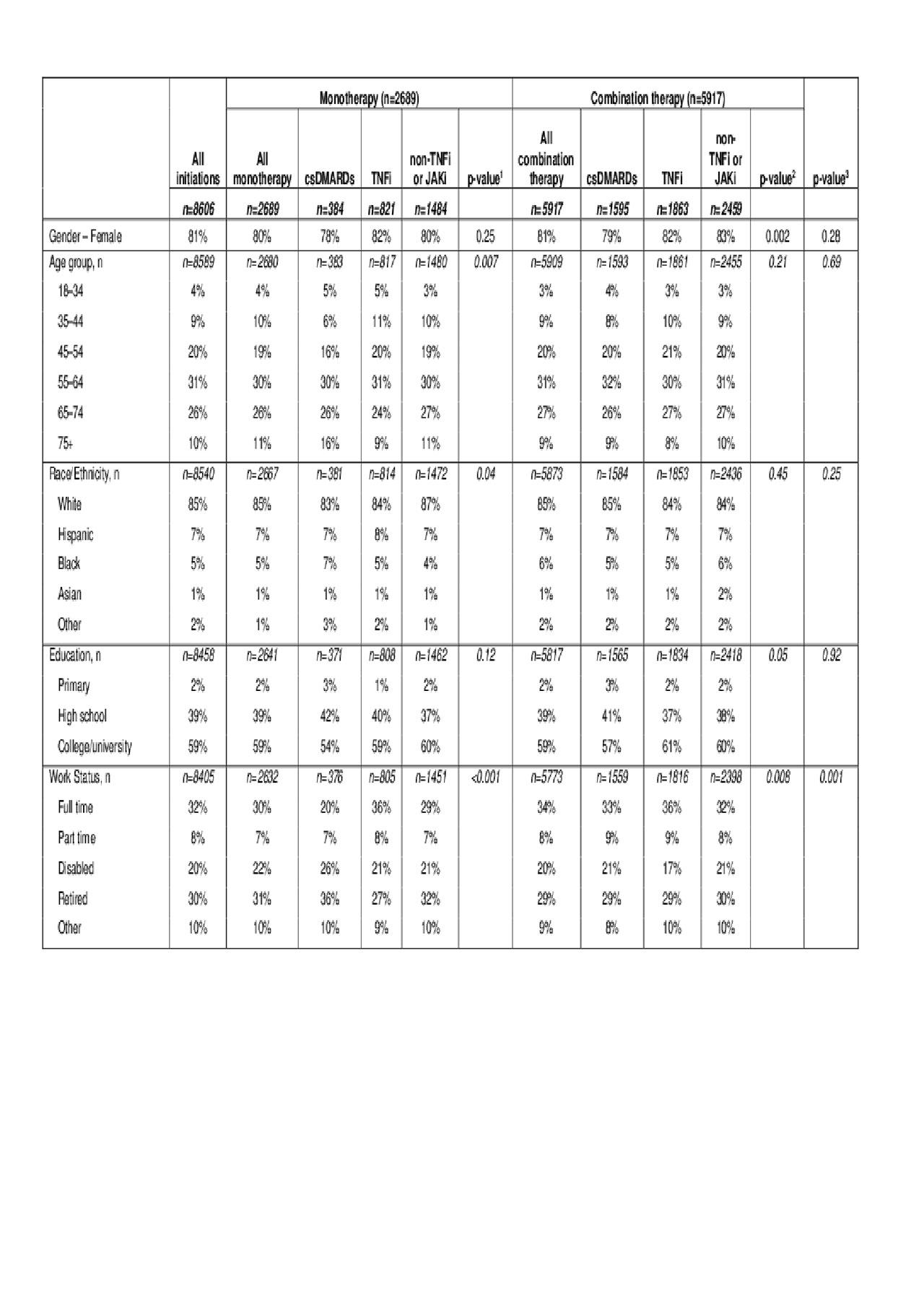
Results: Within 5616 bDMARD-experienced patients, 8606 DMARD initiations met study criteria. Most patients were female (81%) and younger than 65 years of age (Table 1).
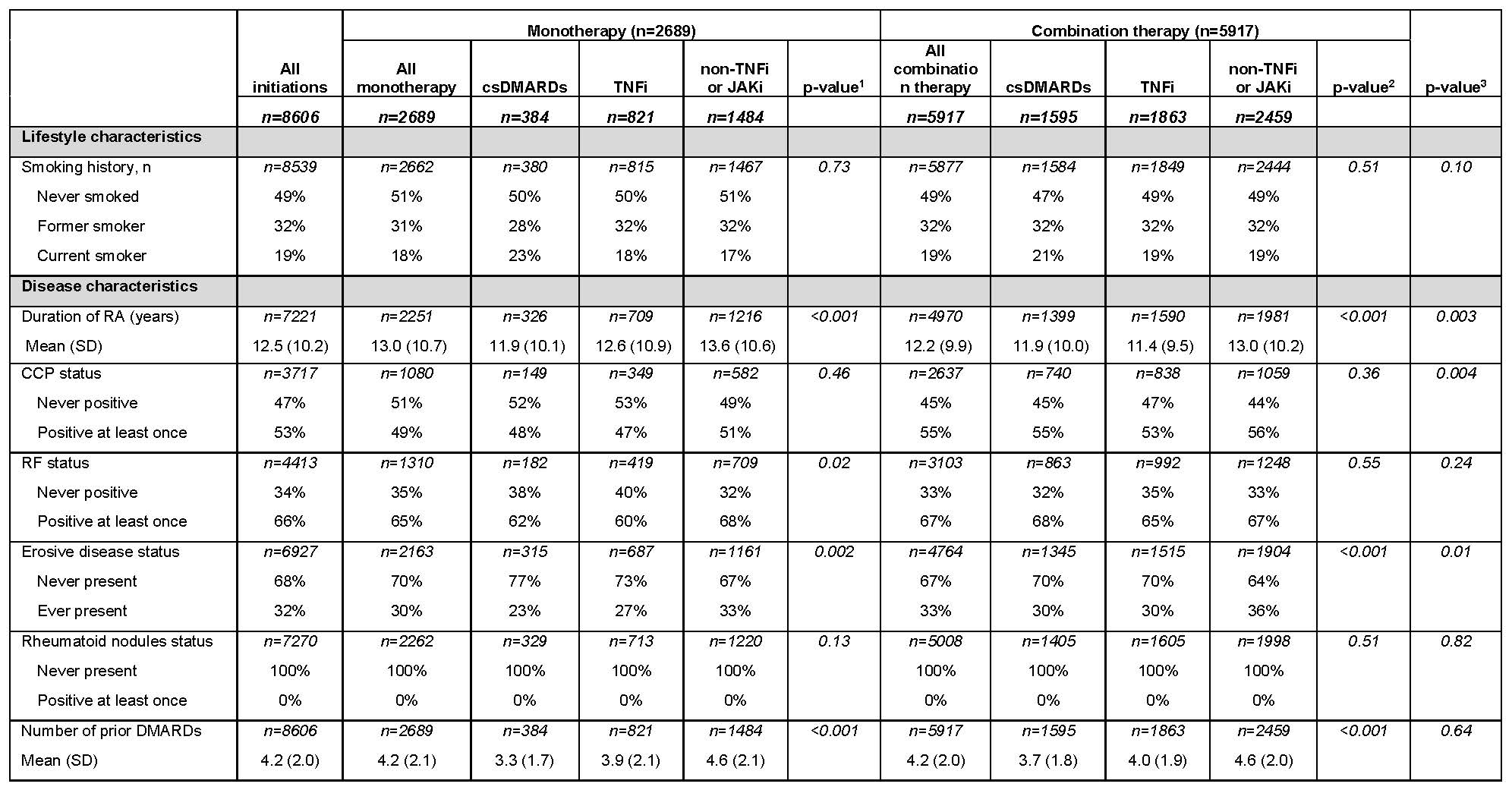
The index treatment regimens were combination therapy (68.8%) or monotherapy (31.2%). Among monotherapies, csDMARDs comprised 14% and TNFi 31%; among combination therapies, csDMARD comprised 27% and TNFi 31%. In monotherapy, the mean RA disease duration was shortest in csDMARD (11.9 years) and longest in non-TNFi or JAKi (13.6 years); similar trends emerged among combination therapies—11.9 years (csDMARD) and 13.0 years (non-TNFi or JAKi; Table 2).
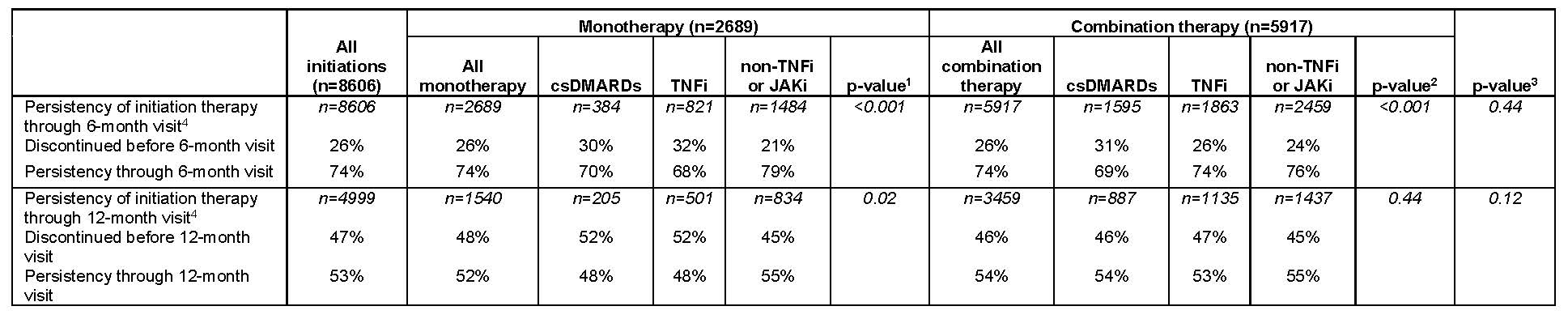
The overall 6-month treatment persistency was 74%, similar in both monotherapy and combination therapy. At 12 months, 52% of patients on monotherapy and 54% of those on combination therapy persisted on their index treatment (Table 3). Based on unadjusted analyses, persistency varied based on drug class among monotherapies at both 6 months (non-TNFi or JAKi 79%, csDMARDs 70%, TNFi 68%) and 12 months (non-TNFi or JAKi 55%, csDMARD 48%, TNFi 48%). Similar results were seen among combination therapies at 6 months (non-TNFi or JAKi 76%, csDMARDs 69%, TNFi 74%) and 12 months (non-TNFi or JAKi 55%, csDMARD 54%, TNFi 53%; Table 3).
Conclusion: In bDMARD-experienced patients, demographic and clinical characteristics varied depending on the type of next treatment, most of which were combination therapy. csDMARD patients demonstrated low (48%–54%) 12-month persistence, suggesting a substantial unmet need in this patient population. Persistency differed among the different drug groups both for monotherapy and combination therapy. Clinicians need to closely monitor patients and revise therapy as needed in this patient population.
csDMARD, conventional synthetic DMARD; JAKi, Janus kinase inhibitors, TNFi, tumor necrosis factor inhibitors.
csDMARD, conventional synthetic DMARD; JAKi, Janus kinase inhibitors, SD, standard deviation; TNFi, tumor necrosis factor inhibitors.
bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; JAK, Janus kinase; JAKi, JAK inhibitors, SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
1- P-value compares the difference among the 3 monotherapies.
2- P-value compares the difference among the 3 combination therapies.
3- P-value compares the difference between all monotherapies and combination therapies.
4- The 6-month visit is defined as a follow up visit occurring ≥3- and ≤9-months following initiation, and the 12-month visit is defined as a follow up visit occurring >9 and ≤15 months following initiation. Based on the cohort inclusion criteria, all patients have a 6-month visit but not all patients have a 12-month visit, which is reflected in the lower sample size for persistency through the 12-month visit.
Note 1: p-values were calculated based on Fisher’s exact tests for categorical variables; the Kruskal-Wallis tests -3 group comparisons- or Wilcoxon rank sum tests -2 group comparisons- for continuous variables. For ease of interpretation, some tests for categorical variables were calculated based on collapsed groups -eg, Age <55 vs Age ≥55, White vs non-White for race, college/university vs others for education, full-time vs others for work status, and current vs other for smoking-. P-values are presented to aid with establishing patterns; care must be taken since we have not formally accounted for the multiple comparisons. Note 2: Note that discontinuation is defined as discontinuation of the index DMARD, no matter whether the patient added another DMARD following initiation of the index DMARD. This is particularly relevant for index csDMARD initiations, because patients may add a bDMARD/JAK while continuing to receive the index csDMARD. In contrast, for index bDMARD initiations, patients will generally need to discontinue the index bDMARD in order to switch to a different bDMARD.
To cite this abstract in AMA style:
Dore R, Antonova J, Harrold L, Chang L, Scherer E, Cronin A, Emeanuru K, Kremer J. Patient Characteristics, Treatment Patterns, and Treatment Persistency in Biologic DMARD-Experienced Rheumatoid Arthritis Patients in a US RA Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/patient-characteristics-treatment-patterns-and-treatment-persistency-in-biologic-dmard-experienced-rheumatoid-arthritis-patients-in-a-us-ra-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-characteristics-treatment-patterns-and-treatment-persistency-in-biologic-dmard-experienced-rheumatoid-arthritis-patients-in-a-us-ra-registry/