Session Information
Date: Monday, November 11, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Treatments, Outcomes, & Measures
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Filgotinib (FIL) is an orally administered, selective inhibitor of Janus Kinase 1 (JAK1) that is under development for the treatment of RA and other inflammatory diseases. The safety and efficacy of FIL has been investigated in the FINCH clinical program that includes three Phase 3, randomized, multicenter studies in patients (pts) with moderate to severely active RA, who had an inadequate response to MTX (FINCH 1; NCT02889796); who were receiving conventional DMARDs and had an inadequate response to biological therapies (FINCH 2; NCT02873936); or who were MTX naïve and initiating MTX alone or in conjunction with FIL or receiving FIL monotherapy (FINCH 3; NCT02886728). Here we present pooled safety data from the double-blind, active and placebo controlled periods of FINCH 1–3 up to 24 weeks.
Methods: The FINCH studies enrolled pts who had a diagnosis of RA (2010 ACR/EULAR criteria) and had ≥ 6 swollen joints and ≥ 6 tender joints at both screening and Day 1. Safety analyses included pts who had received at least one dose of study drug. Pts in FINCH 1 and 2 who did not experience at least a 20% improvement in both swollen joint count and tender joint count by Week 14 discontinued study drug and switched to standard of care. Week 24 safety data from the FINCH 1, 2, and 3 were aggregated and summarized by the number and percentage of pts with events or abnormalities for categorical values. The key safety endpoints reported are treatment-emergent adverse events (AE), treatment-emergent serious AEs, treatment-emergent AEs of interest, all death and treatment-emergent laboratory abnormalities.
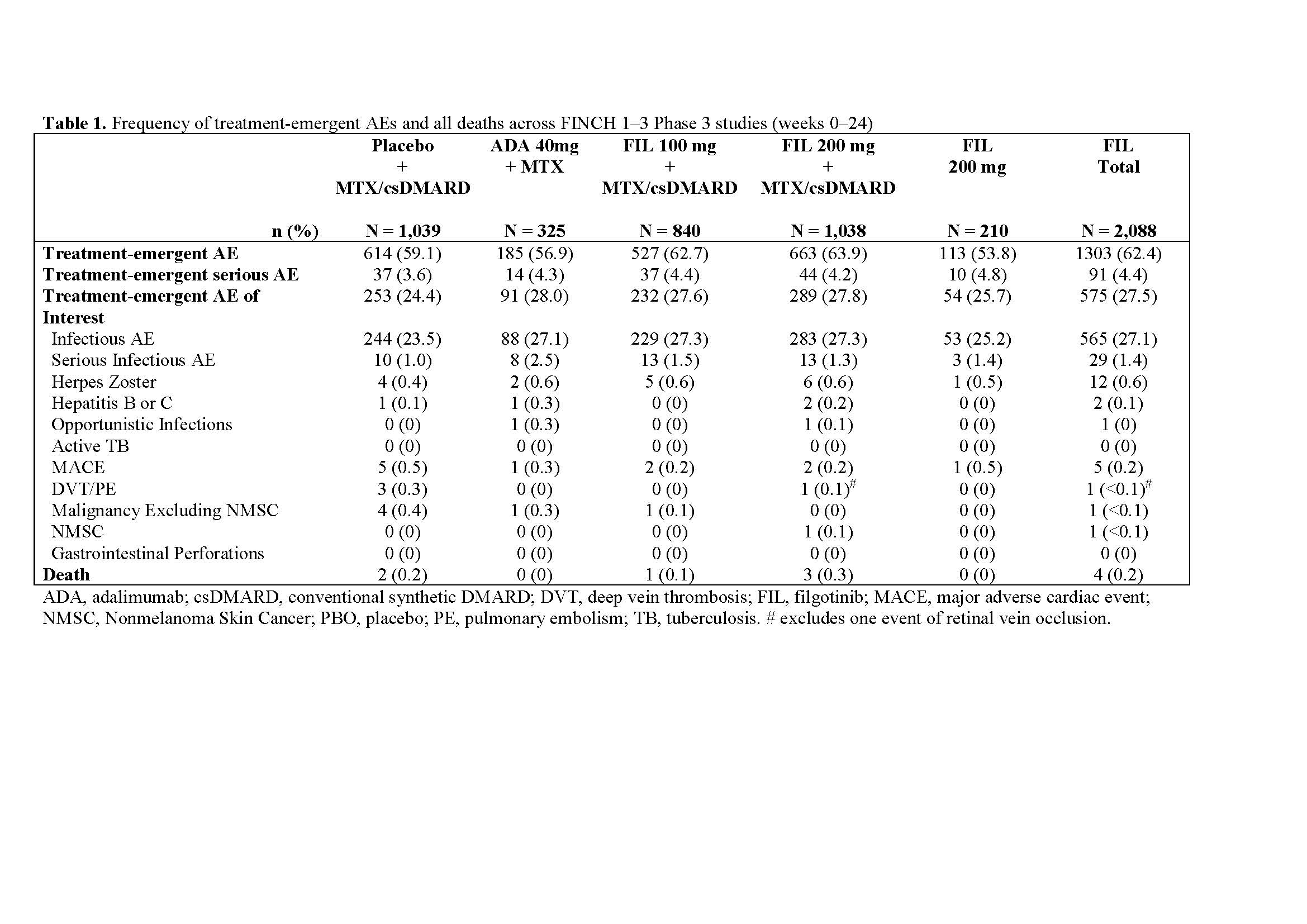
Results: This pooled safety analyses assessed 3,452 pts across FINCH 1–3, including 2,088 pts who received FIL. At Week 24, the frequency of treatment-emergent AEs were similar between pts who received FIL and those in the control arms of the FINCH studies (Table 1). Furthermore, the proportions of pts with treatment-emergent AEs of interest were also similar across groups. The most common treatment-emergent AEs were infections, notably upper respiratory tract and nasopharyngitis. Laboratory abnormalities occurred at similar rates with FIL and placebo or active control and were mostly mild to moderate (Grade 1 & 2). Overall, the frequency of major adverse cardiac events (MACE), herpes zoster virus, deep vein thrombosis (DVT) and pulmonary embolism (PE) was low, and similar across groups. The incidences of MACE were 0.2% for FIL, 0.3% for adalimumab (ADA), and 0.5% for placebo/csDMARD. Additionally, the incidences of DVT/PE were < 0.1% for FIL, 0% for ADA, and 0.3% for placebo/csDMARD.
Conclusion: Although gathered over a short duration (24 weeks), pooled data from this large safety database describing a broad population of pts with RA highlights the favorable safety and tolerability profile of FIL in pts with RA both as a monotherapy and in conjunction with MTX/csDMARD.
To cite this abstract in AMA style:
Winthrop K, Genovese M, Combe B, Tanaka Y, Kivitz A, Matzkies F, Bartok B, Ye L, Guo Y, Tasset C, Sundy J, Keystone E, Westhovens R, Rigby W, Burmester G. Pooled Safety Analyses from Phase 3 Studies of Filgotinib in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pooled-safety-analyses-from-phase-3-studies-of-filgotinib-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pooled-safety-analyses-from-phase-3-studies-of-filgotinib-in-patients-with-rheumatoid-arthritis/