Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient-reported outcomes (PROs) in knee osteoarthritis (OA) trials have been difficult to accurately measure due to heterogenous sources of pain (e.g., from comorbid conditions or joint structure damage) which confound subjects’ symptom discrimination. To improve the measurement accuracy of changes in PROs due to knee OA treatments, trial inclusion criteria must be optimized. Lorecivivint (LOR) is a CLK/DYRK1A inhibitor that modulates the Wnt pathway and is a potential disease-modifying knee OA drug. A post hoc analysis from a 24-week Phase 2b trial assessed the effects of LOR on PROs in subjects without comorbid pain and with baseline medial joint space width (mJSW) 2-4 mm, limiting JSW heterogeneity. Results from the selected Phase 3 dose of 0.07 mg LOR are reported here.
Methods: Knee OA subjects with KL grades 2-3 and Pain Numeric Rating Scale (NRS, [0-10]) ≥4 and ≤8 in the target knee and < 4 in the contralateral knee received a single, 2mL, IA injection of 0.03 mg, 0.07 mg, 0.15 mg, or 0.23 mg LOR or vehicle placebo (PBO) in the target knee at baseline. Positioned, fixed-flexion radiographs were captured at baseline with fixed-location mJSW measured by a central, treatment-blind reader. WPI was collected at screening with pre-specified enrollment of 80% of (WP-, Widespread Pain Index [WPI] ≤ 4, and Symptom Severity Score Question 2 ≤ 2) subjects. PRO endpoints included change from baseline in weekly average of daily OA target knee pain by NRS, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain [0-100], WOMAC Physical Function [0-100], and Patient Global Assessment (PtGA) (VAS [0-100]). Point estimates and effect sizes of the Full Analysis Set (FAS, all dosed subjects) compared to a post hoc completer analysis of subjects with baseline mJSW 2-4 mm who were WP- are reported.
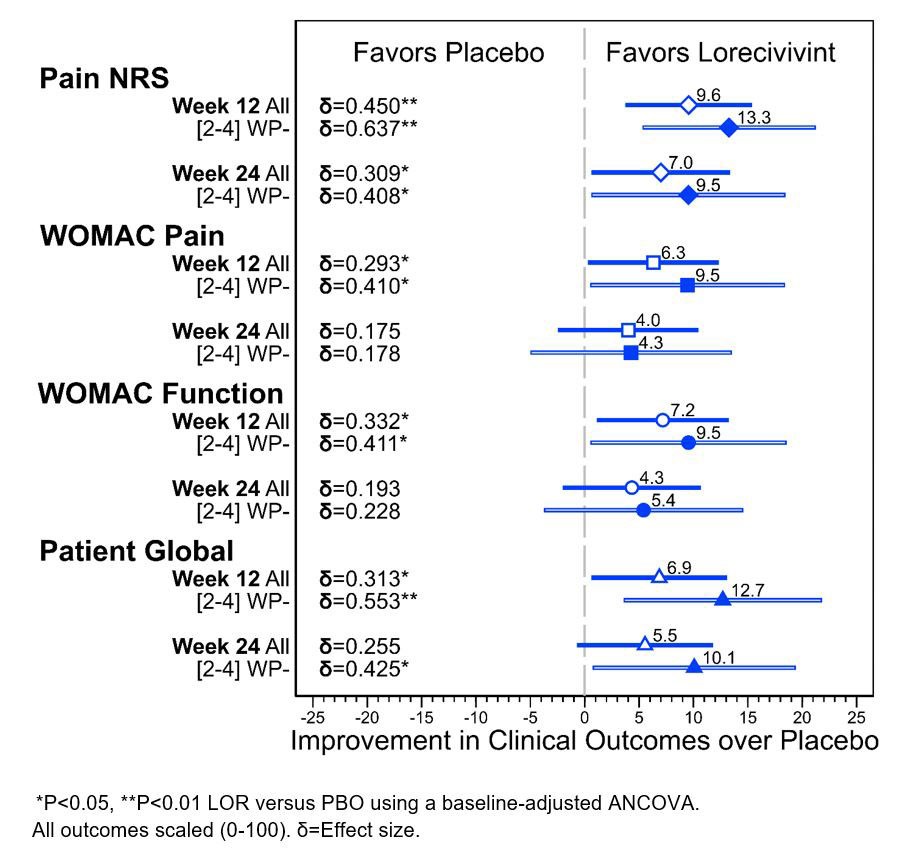
Results: 635 subjects (91.4%) completed the study (mean age 59.0 [±8.5] years, BMI 29.0 [±4.0] kg/m2, female 58.4%, KL3 57.3%). In both FAS and mJSW 2-4 mm who were WP- subjects, significant improvements compared to PBO (P< 0.05) were seen in Pain NRS, WOMAC Pain, WOMAC Function, and PtGA for the 0.07 mg LOR dose group at Week 12 (Figure 1).The effect sizes were improved in the mJSW 2-4 mm who were WP- group in comparison to the FAS for the 0.07 mg LOR dose at Weeks 12 and 24.
Conclusion: In this post hoc analysis of LOR-treated knee OA subjects, those with baseline mJSW 2-4 mm without widespread pain showed improved PRO effect sizes compared to those in the Full Analysis Set beyond the initial significant improvements seen in the Full Analysis Set. These increased point estimates and confidence intervals may help to improve power calculations and create more homogenous populations to test new therapies.
To cite this abstract in AMA style:
Kennedy S, Swearingen C, Tambiah J, Clauw D, Conaghan P. Subject Enrichment Criteria for Phase 3 Studies of Lorecivivint (SM04690), a Potential Disease-Modifying Knee Osteoarthritis Drug: A Post Hoc Study on the Effects of Baseline Comorbid Pain and Joint Space Width on Patient-Reported Outcomes [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/subject-enrichment-criteria-for-phase-3-studies-of-lorecivivint-sm04690-a-potential-disease-modifying-knee-osteoarthritis-drug-a-post-hoc-study-on-the-effects-of-baseline-comorbid-pain-and-joint-s/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subject-enrichment-criteria-for-phase-3-studies-of-lorecivivint-sm04690-a-potential-disease-modifying-knee-osteoarthritis-drug-a-post-hoc-study-on-the-effects-of-baseline-comorbid-pain-and-joint-s/