Session Information
Date: Monday, November 11, 2019
Title: Metabolic & Crystal Arthropathies Poster II: Clinical Trials & Basic Science
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Uric acid homeostasis is determined as a balance between production, intestinal secretion and renal excretion. Around 2/3 of uric acid is excreted by the kidneys, while the remaining 1/3 is excreted by intestine. In people with impaired renal function and hyperuricemia extra-renal elimination ranges between ~50-70% and plays important role in urate homeostasis.1 Existing urate-lowering therapies including oral xanthine oxidase inhibitors, uricosurics, and intravenous uricase agents have limitations either in efficacy or tolerance which contribute to refractoriness. ALLN-346 is an orally administered, non-absorbed, recombinant urate degrading enzyme designed to degrade urate secreted in the intestinal tract and thereby reduce hyperuricemia. Previously, we demonstrated in a urate oxidase KO mouse that 7d of ALLN-346 oral therapy reduces hyperuricemia to the similar extent as allopurinol.2 Here, we tested whether enteral administration of ALLN-346 to pigs with acute hyperuricemia3 could reduce plasma urate (pUA) and urine urate excretion.
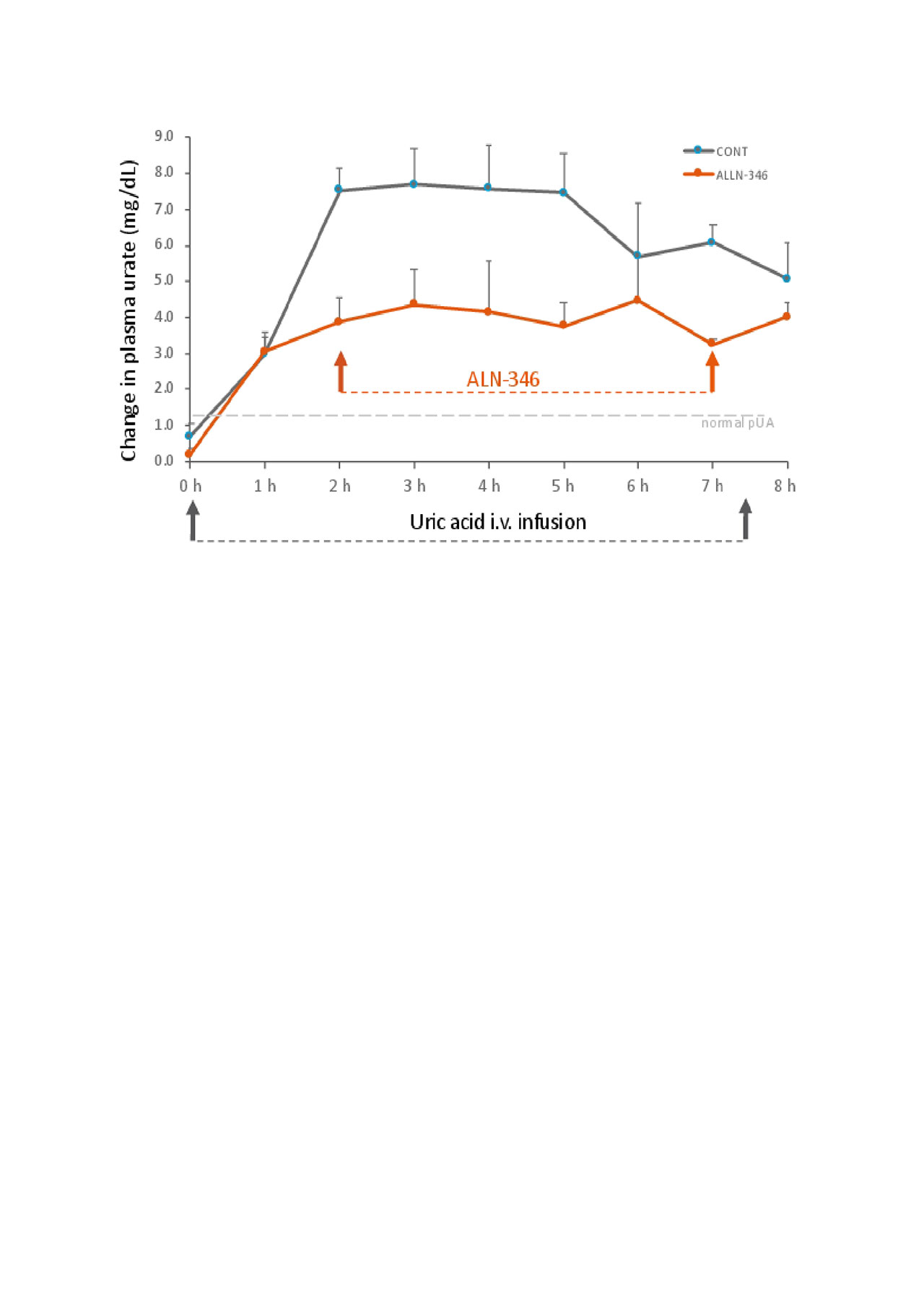
Methods: 7 juvenile pigs (mean bw 17.3kg) with normal kidney function, and with jugular vein catheters for uric acid infusion and blood collection, were administered with ALLN-346 via a duodenal port following induction of hyperuricemia with uric acid infusion. Study was done in pigs that were fed 3x/d (1.0% bw/meal) and watered ad lib. A control run of hyperuricemia was performed by chronic i.v. infusion of uric acid via jugular catheter (10 mg/kg from 40 mg/mL uric acid in 40% glucose) for 7.5h. After 36h wash out, a treatment with ALLN-346 was performed 2h after start of uric acid infusion, when hyperuricemia was induced. ALLN-346 was given hourly to duodenum as a bolus suspension in saline (6 x 10,560u). To estimate changes in pUA, blood was collected at baseline and hourly during the 8h experiment, and AUC0-8h was calculated. Urine was collected for uric acid analysis. Intestinal samples were analyzed for expression of ABCG2 urate transporter, that regulates serum UA, by Western Blot (WB) with pig anti-BCRP/ABCG2 antibody (Abcam).
Results: pUA at baseline was < 1 mg/dL. Uric acid infusion resulted in an immediate increase in pUA, with mean Cmax of 7.7 mg/dL at 3h, and calculated AUC0-8h of 44.3 mg*h/dL. Administration of ALLN-346 attenuated the rise in pUA with Cmax reduced to 4.5 mg/dL and AUC0-8h of 27.5mg*h/dL. The difference between the treatment and control run in pUA was 38% (p=0.0009). The urine uric acid excretion was reduced 16% with ALLN-346 compared to control run. WB confirmed expression of ABCG2 in small intestine, being the highest in the jejunum and ileum.
Conclusion: The results of this pilot experiment in pigs with acute hyperuricemia confirm that intestine plays an active role in urate elimination in conditions of severe hyperuricemia and suggests the potential for ALLN-346 acting in the intestinal tract to reduce pUA levels and overall urate burden in people with hyperuricemia and gout. Further studies are planned to confirm these findings.
References
- Sorensen LB. Role of Intestinal tract in the elimination of uric acid. Arthr. (1965)
- Grujic D et al, ACR 2018
- Szczurek P et al. Oral uricase eliminates blood uric acid in the hyperuricemic pig model PLoS One. (2017)
To cite this abstract in AMA style:
Grujic D, Pirzynowska K, Szczurek P, Pierzynowski S, Desphande A, Drahanchuck O, Mosiichuk N, Wolinski J. Enteral Administration of ALLN-346, a Recombinant Urate-degrading Enzyme, Decreases Serum Urate in a Pig Model of Hyperuricemia [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/enteral-administration-of-alln-346-a-recombinant-urate-degrading-enzyme-decreases-serum-urate-in-a-pig-model-of-hyperuricemia/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enteral-administration-of-alln-346-a-recombinant-urate-degrading-enzyme-decreases-serum-urate-in-a-pig-model-of-hyperuricemia/