Session Information
Date: Monday, November 11, 2019
Title: Metabolic & Crystal Arthropathies Poster II: Clinical Trials & Basic Science
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Gout is caused by the deposition of monosodium urate (MSU) crystals in joints due to chronic hyperuricemia. Long-term treatment focuses on reducing serum uric acid (sUA) levels, thus allowing MSU crystals to dissolve. Administration of uricase, a non-human enzyme that catalyzes the conversion of uric acid into the water-soluble allantoin, has emerged as a therapy for the treatment of patients with gout that is refractory to conventional treatments. Currently approved uricase products are immunogenic, which results in decreased efficacy as anti-drug antibodies (ADAs) develop as well as increased safety concerns. SEL-212 is a novel combination product consisting of pegadricase co-administered with proprietary ImmTOR tolerogenic nanoparticles. ImmTOR has been designed to mitigate the formation of ADAs to pegadricase by inducing tolerogenic dendritic cells and antigen-specific regulatory T cells. Mitigating the formation of ADAs is vital in maintaining a sustained control of sUA levels in patients. Here we describe the results of a Phase 2 clinical trial (NCT02959918) designed to identify the appropriate doses of the 2 components for further development of SEL-212.
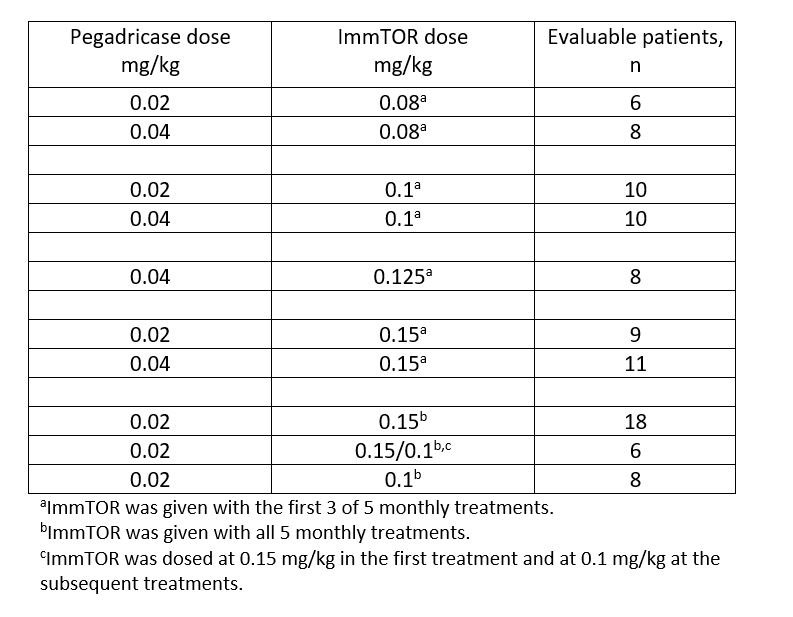
Methods: In this open-label dose-ranging study, patients with symptomatic gout (≥1 tophus, gout flare within previous 6 mo, and/or gouty arthropathy) and hyperuricemia (sUA ≥6 mg/dL) were enrolled and treated with pegadricase at 0.2 or 0.4 mg/kg alone, or combined with 0.05 to 0.15 mg/kg ImmTOR. Treatments were administered intravenously monthly for 3 cycles of SEL-212, followed by 2 additional cycles with pegadricase alone or SEL-212 (see Table). Dosing was stopped in patients demonstrating sUA ≥1.0 mg/dL on Day 21 after the previous dose. Safety, tolerability, sUA, ADAs, and clinical outcomes were monitored throughout the study.
Results: A total of 152 patients enrolled in the study. The average age was 54.8 (range 23-75) years, most were male (90.8%), and the mean duration of symptomatic gout was 8.0 years. When administered alone at either dose, pegadricase resulted in a rapid reduction in sUA, which was not sustained due to ADA formation in 5 of 6 patients. The addition of ImmTOR at ≥0.1 mg/kg reduced ADA formation, allowing sustained reduction in sUA, but these responses were attenuated when ImmTOR was removed during cycles 4 and 5. The combination of pegadricase (0.2 mg/kg) and ImmTOR (0.1 or 0.15 mg/kg) was given during all 5 cycles in 3 additional cohorts. In these cohorts, 66% of evaluable patients (21/32) maintained sUA levels below 6 mg/dL at Week 20 after 5 monthly doses of SEL-212, and 100% of patients who had sUA < 6 mg/dL at 12 weeks maintained control through 20 weeks. Sustained reduction of sUA levels correlated with low or no ADAs. The percentage of patients experiencing flares in these 3 cohorts declined from 35% during month 1 to 9%-10% during months 3, 4, and 5.
Conclusion: Based on these results, the combination of pegadricase (0.2 mg/kg) and ImmTOR (0.15 mg/kg) were selected for use in a head-to-head comparison study of SEL-212 to pegloticase, a pegylated uricase currently FDA approved for use in patients with treatment-refractory gout (NCT03905512).
To cite this abstract in AMA style:
Azeem R, Kivitz A, Plotkin H, Johnston L, Kishimoto T, Park J, Smolinski S, DeHaan W. Phase 2 Dose-ranging Study of SEL-212 in Symptomatic Gout Patients: Selection of Doses for Further Clinical Development [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/phase-2-dose-ranging-study-of-sel-212-in-symptomatic-gout-patients-selection-of-doses-for-further-clinical-development/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phase-2-dose-ranging-study-of-sel-212-in-symptomatic-gout-patients-selection-of-doses-for-further-clinical-development/