Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Large pragmatic clinical trials (PCTs) are increasingly used to conduct comparative effectiveness research (CER). PCTs typically have simple inclusion/exclusion criteria and hard outcomes (e.g. hospitalized infection, death) but can be challenging to conduct due to the large number of patients required. In the context of planning a safety PCT of the live zoster vaccine in older rheumatoid arthritis (RA) patients receiving anti-TNF therapy, we evaluated the use of various databases to assess the feasibility of recruiting the 4,000 patients needed for the trial (based upon sample size calculations required for 80% power) and to facilitate rheumatology site selection.
Methods: Using multiple health plan and registry databases (e.g. 100% sample of Medicare patients in 2009; younger RA patients enrolled in a commercial insurance health plan), we identified RA patients age >= 50 on the basis of physician diagnoses and receipt of received anti-TNF therapy in the last 90 days of 2009. Extrapolations were made to estimate the prevalence of anti-TNF use for patients whose pharmacy coverage data was not present in the available databases.
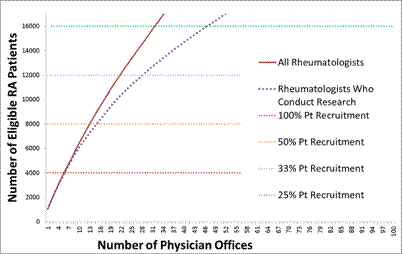
These eligible individuals were linked to individual rheumatologists (e.g. using National Provider Identification numbers) and cross referenced against multiple sources of information (e.g. FDA 1572 registry, known participation in any U.S. RA registry or consortium) that ) that allowed identification of rheumatologists with research experience. Rheumatologists were grouped together into practices (offices) using billing information, and offices were sorted by size based upon having the greatest to the least number of eligible patients. The number of rheumatologist offices needed to fully recruit to the needed sample size of 4,000 older RA patients on anti-TNF therapy was plotted as a function of the hypothesized patient recruitment rate (e.g. 25%, 33%, 50%).
Results: More than 150,000 RA patients receiving anti-TNF therapy at the end of 2009 were identified and grouped into the rheumatologists’ offices at which they received care. The number of eligible RA patients was plotted for the largest 100 rheumatologists’ offices (Figure, solid line). A majority of the largest offices had evidence that they participated in research, as evidence by the dotted line being almost superimposed with the solid line for the first 6000+ patients). Even with a participation rate that was < 30%, fewer than 40 rheumatologist offices with a prior history of clinical research would be required in order to successfully recruit the proposed PCT.
Conclusion: Large health plan and registry databases appear useful to assess feasibility of large pragmatic trials and to assist in selection of rheumatology sites with the greatest number of eligible patients. This novel approach is applicable to trials with simple inclusion/exclusion criteria that can be readily assessed in these data sources.
Disclosure:
J. R. Curtis,
Roche/Genetech, UCB< Centocor, Corrona,Amgen, Pfizer, BMS, Crescendo, Abbott,
2,
Roche/Genetech,UCB, Centocor, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abbott,
5;
L. Chen,
None;
F. Xie,
None;
J. Zhang,
None;
K. G. Saag,
AHRQ, NIH/NIAMS,
2,
Amgen;Abbott;Ardea:Lilly:Merck:Novartis:Regeneron:Savient:URL,
5,
NOF;ACR,
6;
S. Cofield,
Teva Neruoscience, Centocor Ortho-Biotech Svcs LLC, Medimmune, american Shoulder and Elbow Society, Consortium of Multiple Sclerosis Centers, and Pythagorus, Inc,
5;
K. L. Winthrop,
Pfizer Inc,
5,
Pfizer Inc, UCB, Abbott, Amgen,
2;
N. C. Wright,
Amgen,
2;
E. S. Delzell,
Amgen,
2.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-health-plan-data-to-assess-feasibility-of-large-pragmatic-clinical-trials-in-rheumatoid-arthritis/