Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Patients with rheumatic diseases given immunosuppressive therapy are susceptible to various types of infections, especially pulmonary infections that affect their vital prognosis. The aim of this large-scale, multi-center, prospective cohort study was to identify risk factors for development of pulmonary infection in patients receiving immunosuppressive treatment for rheumatic diseases (PREVENT study).
Methods: We enrolled those patients who were admitted to participating hospitals for treatment of rheumatic diseases and started immunosuppressive therapy with corticosteroids, conventional immunosuppressants, or biologics. Observation was stopped either 12 months after enrollment, or the day a patient developed predefined pulmonary infection or was lost-to- follow up, whichever came first. The validity of the diagnosis for pulmonary infection was assessed by an event-monitoring committee. We collected demographic data and clinical data for rheumatic diseases at baseline, data for candidate risk factors for pulmonary infection at baseline and month 6, and usage of drugs throughout the observation period. Risk factors for pulmonary infection were investigated by univariate and multivariate analyses.
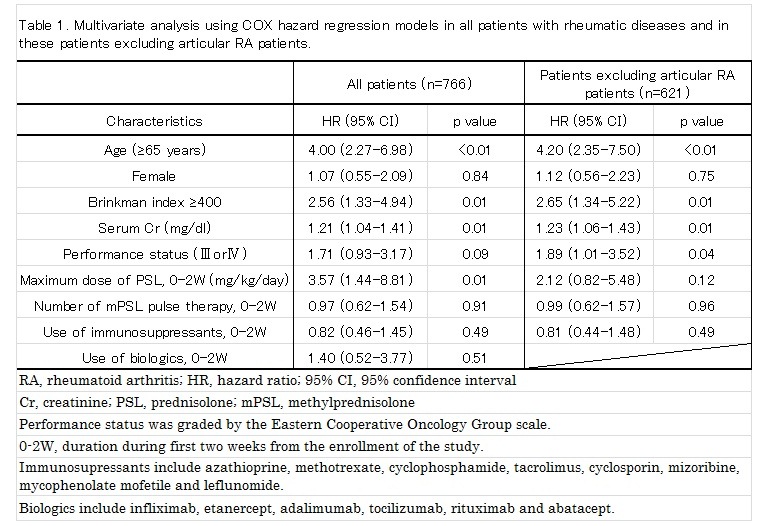
Results: Of 766 patients enrolled, 668 patients (87.2%) were observed for 12 months, 32 patients (4.2%) died, and 66 (8.6%) patients were lost-to-follow up by month 12. Sixty-one patients (8.0%) developed pulmonary infection: bacterial pneumonia, 25; Pneumocystis jirovecii pneumonia, 20; fungal pneumonia, 5; cytomegalovirus pneumonia, 3; tuberculosis, 3; others, 5. Kaplan-Meier curves showed a significantly lower cumulative survival rate in patients with pulmonary infection compared to those without pulmonary infection (p<0.01, log-rank test). Because treatment for RA patients without active extra-articular manifestation (articular RA patients, n=145) was significantly different from that for the rest of the patients, we performed multivariate analyses using COX hazard regression models in all patients (n=766) and in these patients excluding articular RA patients (n=621) (Table 1). Older age (≥65 years-old), higher Brinkman index (≥400), higher serum creatinine (sCr) level, and higher maximum prednisolone dose (mg/kg/day) during the first two weeks of treatment were significantly associated with development of pulmonary infection in all patients. Older age, higher Brinkman index, higher sCr level, and disturbed performance status were significantly associated with development of pulmonary infection in patients excluding articular RA patients.
Conclusion: This is the first large-scale, prospective study identifying risk factors for pulmonary infection in patients with rheumatic diseases in the literature. Prophylactic measures should be taken accordingly for better benefit-risk balance of treatment.
Disclosure:
H. Yamazaki,
None;
R. Sakai,
None;
R. Koike,
None;
Y. Miyazaki,
None;
M. Tanaka,
None;
T. Nanki,
None;
K. Watanabe,
None;
S. Yasuda,
None;
T. Kurita,
None;
Y. Kaneko,
None;
Y. Tanaka,
Bristol-Myers Squibb KK,
2,
MSD KK,
2,
Chugai Pharmaceutical ,
2,
Mitsubishi Tanabe Pharma,
2,
Astellas Pharma ,
2,
Abbot Japan ,
2,
Eisai,
2,
Janssen Pharmaceutical KK,
2,
Mitsubishi Tanabe Pharma ,
8,
Abbot Japan ,
8,
Chugai Pharmaceutical ,
8,
Janssen Pharmaceutica KK,
8,
Santen Pharmaceutical ,
8,
Pfizer Japan ,
8,
Astellas Pharma ,
8,
Daiichi Sankyo ,
8;
Y. Nishioka,
Chugai Pharmaceutical,
2,
Pfizer Japan,
2,
Abbott Japan,
2,
Baistol-Myers Squibb KK,
2;
Y. Takasaki,
None;
K. Nagasaka,
None;
K. Amano,
Chugai Pharmaceutical ,
2,
Abbott Japan ,
2,
Astellas Pharmaceutical ,
2;
S. Tohma,
Pfizer Japan,
2,
Eisai,
2,
Chugai Pharmaceutical,
2;
M. Dohi,
None;
T. Sugihara,
None;
H. Sugiyama,
None;
Y. Kawaguchi,
None;
N. Inase,
None;
S. Ochi,
None;
H. Hagiyama,
None;
N. Miyasaka,
Abbot Japan,
2,
Astellas Pharma,
2,
Chugai Pharmaceutical,
2,
Daiichi Sankyo,
2,
Eisai,
2,
Janssen Pharmaceutical KK,
2,
Mitsubishi Tanabe Pharma,
2,
Taketa Pharmaceutical ,
2,
Teijin Pharma,
2,
Bristol-Myers Squibb KK,
2;
M. Harigai,
Abbott Japan,
2,
Astellas Pharma,
2,
Bristol Myers Squibb KK,
2,
Chugai Pharmaceutical,
2,
Eisai,
2,
Janssen Pharmaceutical KK,
2,
Micsubishi Tanabe Pharma,
2,
Santen Pharmaceutical,
2,
Takeda Pharmaceutical,
2,
Pfizer Japan,
2.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/how-to-assess-risks-for-pulmonary-infection-in-patients-receiving-immunosuppressive-treatment-for-rheumatic-diseases-a-report-from-a-large-scale-prospective-cohort-study/