Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Filgotinib (FIL) is an oral selective Janus kinase (JAK1) inhibitor. In studies to date, FIL has been shown to be effective and safe in patients with RA and other inflammatory diseases (IBD/AS/PsA). DARWIN 3 (NCT02065700) is an ongoing, open-label, long-term extension study of earlier phase 2b studies evaluating the longer-term safety and efficacy of FIL in RA.1,2
Methods: All patients who were inadequate responders to MTX completing the 24-week DARWIN 1 (FIL + MTX) and DARWIN 2 (FIL monotherapy) studies were eligible to enter DARWIN 3. All patients in DARWIN 3 received FIL 200 mg/day, with the exception of 15 males, who received FIL 100 mg/day (7 in FIL + MTX, 8 in FIL). Here we present week 156 interim analysis data from the first dose of FIL in the parent studies through 30 May 2018. Patients were analyzed by FIL + MTX (from DARWIN 1) or FIL monotherapy (from DARWIN 2). The event rate was calculated as the total events/total years of exposure of FIL. If the subjects were continuing the study at the time of analysis, the exposure was calculated up to the data cut date.
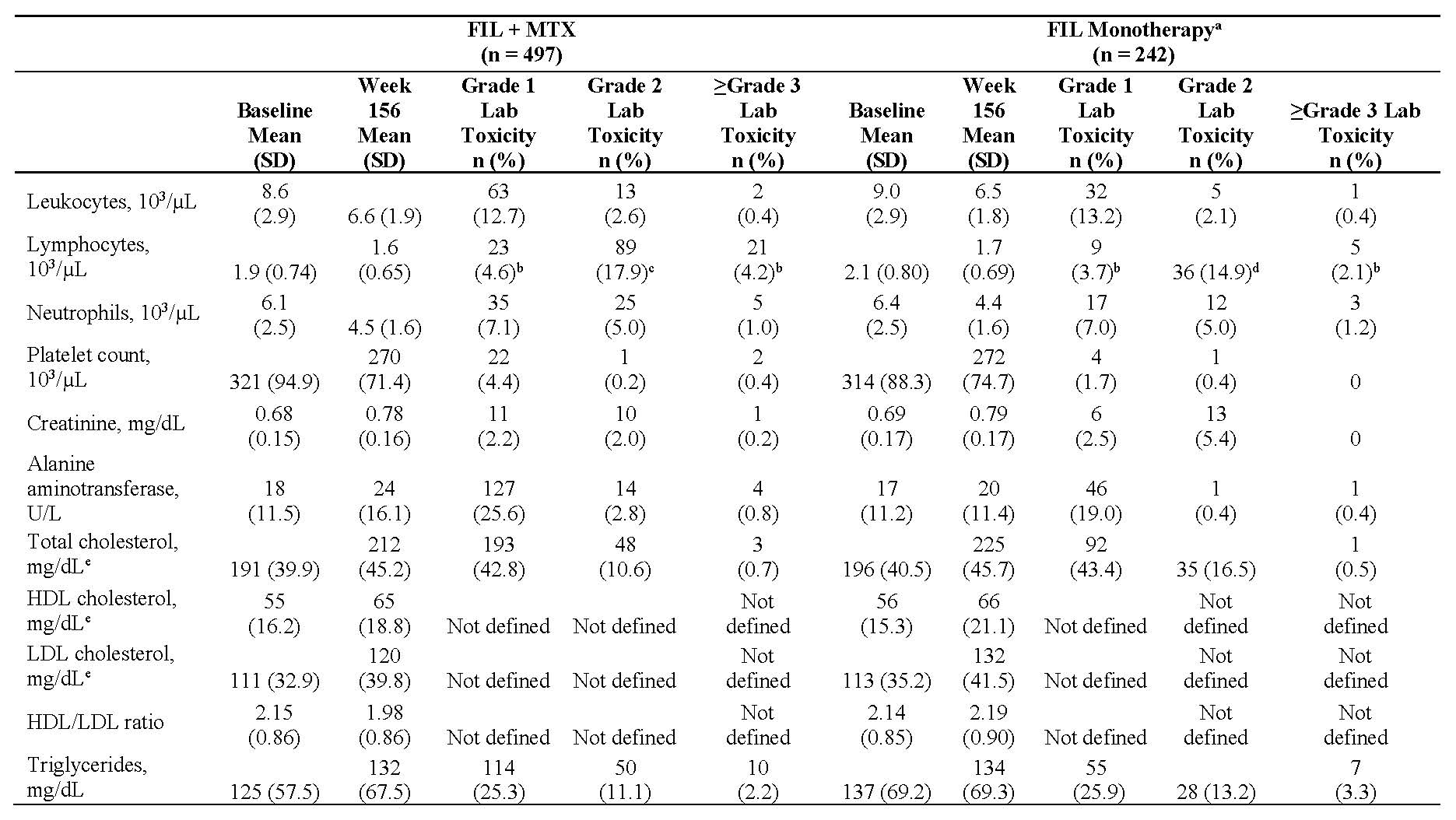
Results: Of 877 patients completing the parent studies, 739 patients enrolled in DARWIN 3 (497 from DARWIN 1, 242 from DARWIN 2); the majority of patients in DARWIN 1 and 2 were female (81.5%, 81.8%) and white (75.3%, 74.8%) with a mean age of 53 and 52 years, respectively. The mean baseline MTX dose in the FIL + MTX group was 16.8 mg/week. At week 156, 59.9% of patients remained on study treatment. The most common reasons for discontinuation were adverse events (26.5%) and subject request (9.1%). Total exposure to FIL was 2203 patient years; mean ± standard deviation (SD) exposure was 2.86 ± 1.21 years for FIL + MTX and 3.04 ± 1.22 years for FIL monotherapy. Treatment-emergent adverse events (TEAEs) occurred in 419 (84.3%) and 203 (83.9%) patients receiving FIL + MTX and FIL monotherapy; serious TEAEs occurred in 45 (9.1%) and 33 (13.6%), respectively. Event rates of adverse events of special interest remained low at week 156 (Table 1). There were 5 deaths (meningococcal meningitis, pneumonia, non-Hodgkin’s lymphoma [2], and deep vein thrombosis with pulmonary embolism); none of which occurred after the week 132 analysis (2 in FIL + MTX; 3 in FIL monotherapy). Laboratory values are presented in Table 2. Clinical efficacy up to week 156, as measured by ACR20/50/70 responses, and DAS28(CRP) ≤3.2 and DAS28(CRP) < 2.6 using observed cases are shown in Figure 1.
Conclusion: FIL was generally well tolerated. No new safety signals emerged. There was no safety difference in patients receiving combination therapy with MTX vs filgotinib monotherapy. Efficacy could be sustained up through week 156 in both the monotherapy and MTX combination groups. Clinical correlations with laboratory value changes are being explored and will be presented at the meeting.
References
- Westhovens R, et al. Ann Rheum Dis 2017;76:998-1008.
- Kavanaugh A, et al. Ann Rheum Dis 2017;76:1009-1019.
a- For week 132, subjects who received MTX for at least 50% of the time while they were receiving FIL in parent study and DARWIN 3, subjects were counted in FIL+ MTX group.
b- For week 156, all patients from DARWIN 1 were counted as FIL + MTX and from DARWIN 2 were counted as FIL monotherapy.
c- 19 patients in DARWIN 2 started MTX during DARWIN 3 but are categorized as FIL monotherapy.
d- Week 132 interim analysis includes adverse events occurring during placebo/screening period, pre-FIL dose. Week 156 interim analysis only includes adverse events that began on or after FIL start date.
FIL, filgotinib; NMSC, nonmelanoma skin cancer; PYE, patient-years of exposure.
All treatment-emergent lab toxicity events were in the same direction as the change from baseline to week 156 except where shown.
a- 19 patients in DARWIN 2 started MTX during DARWIN 3 but are categorized as FIL monotherapy.
b- Decreased lymphocytes; no increases reported.
c- Patients with decreased lymphocytes; 34 -6.8%- patients had Grade 2 increased lymphocytes.
d- Patients with decreased lymphocytes; 25 -10.3%- patients had Grade 2 increased lymphocytes.
e- All lipids reported using fasting data.
FIL, filgotinib; HDL, high-density lipoproteins; LDL, low-density lipoproteins.
FIL, filgotinib.
To cite this abstract in AMA style:
Kavanaugh A, Westhovens R, Winthrop K, Lee S, Greer J, DeZure A, An D, Ye L, Sundy J, Besuyen R, Meuleners L, Alten R, Genovese M. Rheumatoid Arthritis Treatment with Filgotinib: Week 156 Safety and Efficacy Data from a Phase 2b Open-Label Extension Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/rheumatoid-arthritis-treatment-with-filgotinib-week-156-safety-and-efficacy-data-from-a-phase-2b-open-label-extension-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatoid-arthritis-treatment-with-filgotinib-week-156-safety-and-efficacy-data-from-a-phase-2b-open-label-extension-study/