Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is a systemic inflammatory condition associated with increased rates of atherosclerotic progression via pathogenic remodeling of high-density lipoprotein (HDL)-associated proteins, resulting in reduction of HDL function.1 Upadacitinib (UPA), a selective JAK1 inhibitor, has demonstrated efficacy in patients (pts) with moderate-to-severe RA.
We assess UPA treatment on cholesterol efflux capacity (CEC) and evaluate CEC with changes in inflammation and serum lipids.
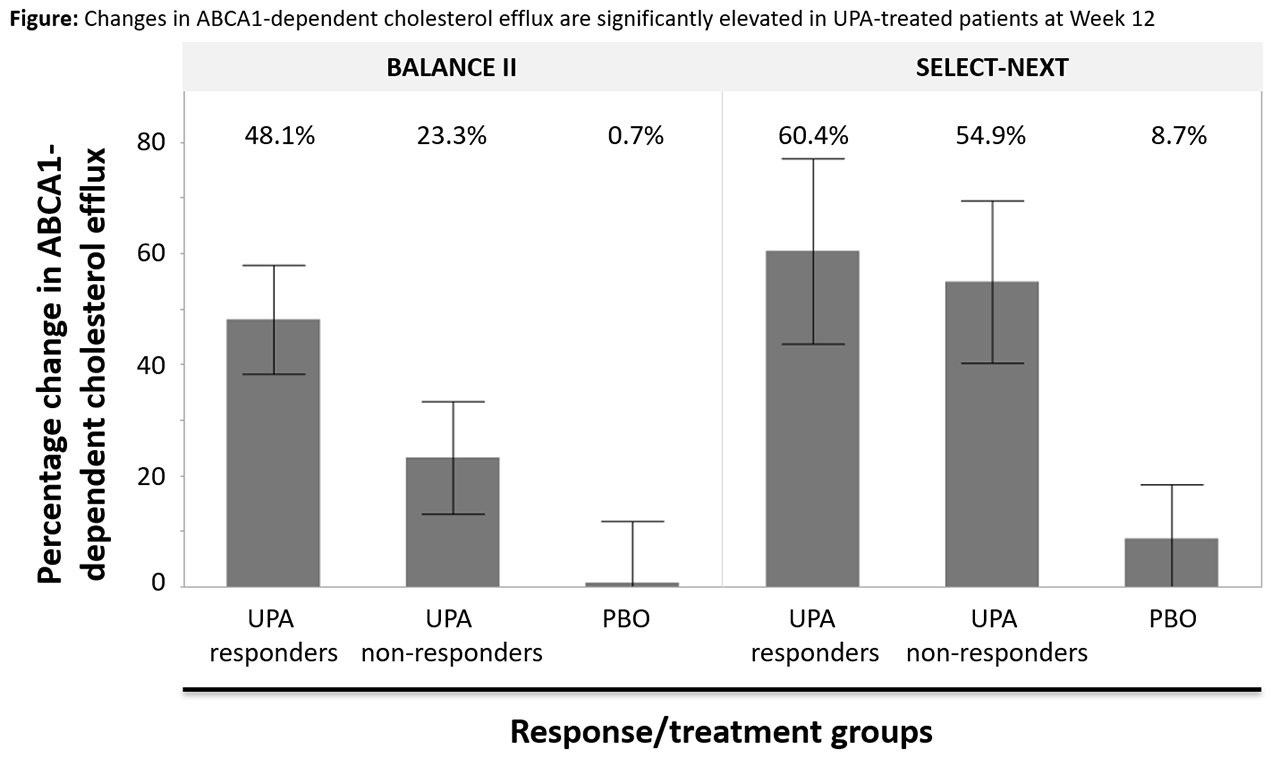
Methods: A subset of pts from the Phase 2 BALANCE II study2 and the Phase 3 SELECT-NEXT study3 were selected from the pool of pts with serum samples available at baseline and Week (Wk) 12. Pts were matched for age and sex, and selected based on level of response to UPA therapy (BALANCE II, UPA 6mg BID: 39 responders [mean change in DAS28-CRP at Wk 12 -3.22] and 30 non-responders [mean change in DAS28-CRP -0.33]; SELECT-NEXT, UPA 15mg QD: 20 responders [mean change in DAS28-CRP -3.78] and 20 non-responders [mean change in DAS28-CRP -0.67]). A demographically similar placebo (PBO) group without selection based on degree of response was also included (20 pts from each study). J774 macrophages labeled with [3H]-cholesterol (treated with cAMP to express ABCA1 or left untreated) were exposed to patient sera. The difference between cholesterol efflux from serum-exposed and unexposed cells provided a measurement of CEC. Results were compared between the responder, non-responder, and PBO groups using Tukey’s mean comparison method; correlations were calculated using the Pearson method; all statistical analyses were performed in JMP 13.10 (SAS Institute).
Results: In both studies, changes in global and ABCA1-dependent CEC, and to a lesser extent non-ABCA1-dependent CEC, were significantly higher in the UPA-treated group compared with the PBO group (Figure). In the BALANCE II study, there was a significant increase in CEC among UPA responders relative to PBO and a numerically apparent difference observed between UPA non-responders and PBO. Notably, in the SELECT-NEXT study, a similar and highly significant improvement in CEC was observed for both UPA-treated groups relative to PBO (without a significant difference between the responder and non-responder groups). Despite the lack of a consistent association between change in CEC and change in clinical disease activity, observed increases in CEC correlated significantly with reductions in CRP levels in all groups across all active treatment groups. Additionally, increases in CEC at Wk 12 correlated well with changes in total blood cholesterol and HDL levels, but weakly with changes in blood low-density lipoprotein (LDL) levels.
Conclusion: UPA treatment is associated with significant improvement in CEC. This effect was observed even among those demonstrating minimal clinical response (but not in those treated with PBO). The effect seems to be primarily driven by ABCA1-dependent cholesterol efflux and is strongly correlated with a rise in HDL cholesterol as well as reduction in systemic inflammation as measured by change in CRP.
1. Charles-Schoeman C, et al. Arthritis Rheumatol 2017;69:46–57;
2. Genovese MC, et al. Arthritis Rheumatol 2016;68:2857–66;
3. Burmester GR, et al. Lancet 2018;391:2503–12
To cite this abstract in AMA style:
Charles-Schoeman C, Sornasse T, Sokolove J. Treatment with Upadacitinib Is Associated with Improvements in Reverse Cholesterol Transport in Patients with Rheumatoid Arthritis: Correlation with Changes in Inflammation and HDL Levels [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-with-upadacitinib-is-associated-with-improvements-in-reverse-cholesterol-transport-in-patients-with-rheumatoid-arthritis-correlation-with-changes-in-inflammation-and-hdl-levels/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-with-upadacitinib-is-associated-with-improvements-in-reverse-cholesterol-transport-in-patients-with-rheumatoid-arthritis-correlation-with-changes-in-inflammation-and-hdl-levels/