Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Allopurinol (ALLO), the leading hypouricemic drug worldwide, exposes to mild (M) and severe (S) cutaneous adverse reactions (CARs). SCARS have been associated with HLA*B-58 01. However, the reported strength of the association has varied across ethnicities and has been little studied in Vietnam. Little is known about risk factors for MCARs.
The aim of this prospective study was to investigate risk factors, including HLA*B-58 01, for MCARs and SCARs in the predominant Kinh ethnicity of Vietnam.
Methods: All patients were Kinh Vietnamese and were prospectively recruited in Ho Chi Minh City, after Ethics committee approval. SCARs were recruited in dermatology and allergy hospital departments, MCARs in the same departments and at the Vien Gut clinic (specialized in gout care), and tolerant gouty patients (no skin reaction after at least 3 months from the last increment in ALLO dose) at the Vien Gut clinic. Clinical data were prospectively collected and HLA*B-58 01 genotype was obtained using the PG5801 DNA detection kit (Pharmigene-Taiwan). Fisher exact test for categorical variables and Kruskal/Wilkonson test for quantitative variables were used for statistics.
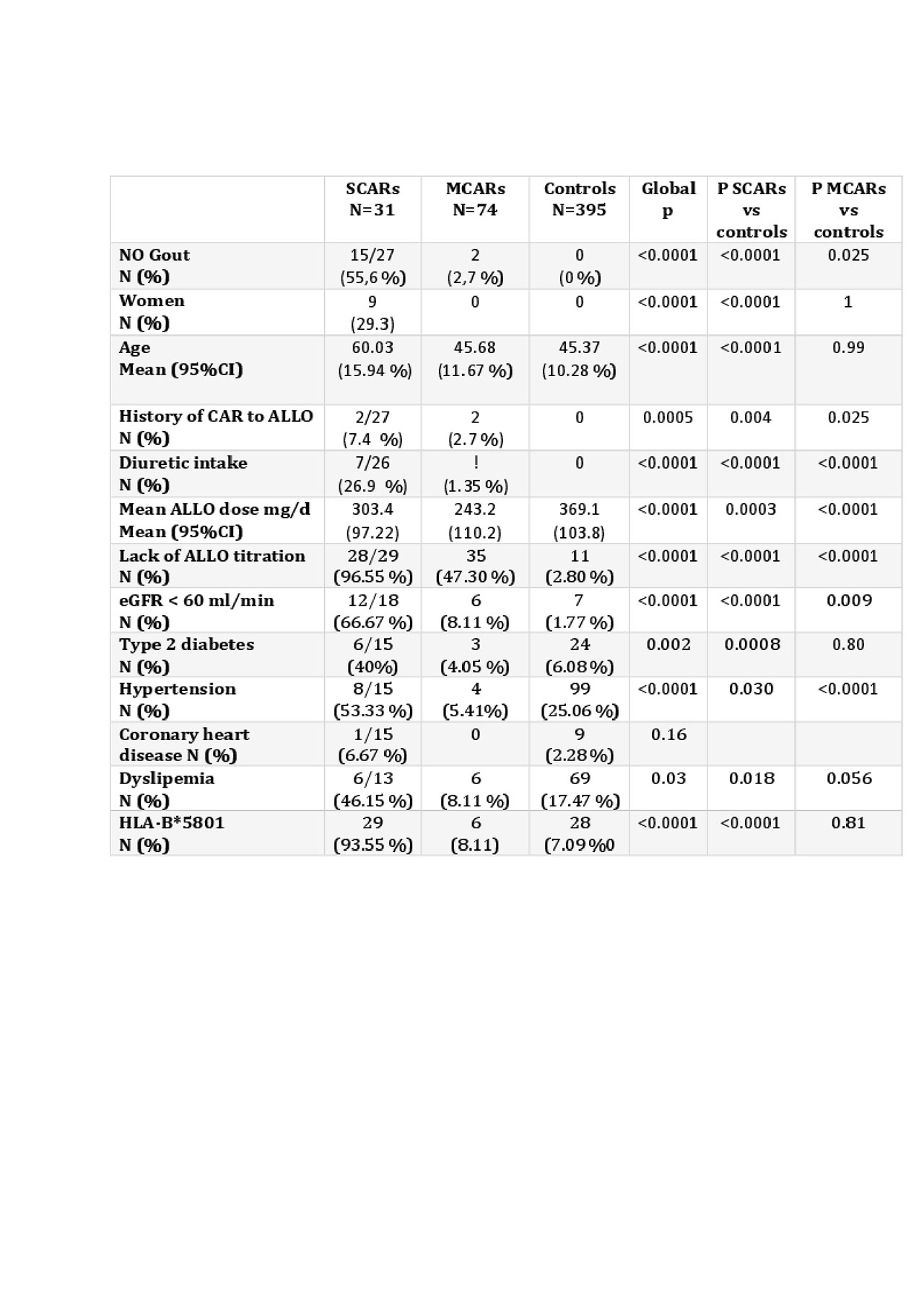
Results: Between March 2017 and May 2019, 31 patients with a non-fatal SCAR. (29 toxic necrotic epidermolysis/ Stevens-Johnson syndrome and 2 DRESS), 74 patients who stopped ALLO because of MCAR and 395 ALLO-tolerant patients were recruited. Table 1 shows the main features of interest in the 3 groups. The Odds ratios of lack of dose escalation, HLA*B-58 01 presence and GFR< 60 ml/min to develop SCARs were calculated at 883 (, 95%CI:128; 4504), 185(95 % CI: 43; 1595) and 104 (95%CI:28;454), respectively.
Conclusion: In the studied Vietnamese Kinh population, lack of ALLO titration was the strongest risk factor for SCARs. HLA*B-58 01 and other known factors, including renal failure but not high ALLO dose, were also significantly associated with SCARs. MCARs associated with lack of ALLO titration and renal failure but not with HLA*B-58 01
To cite this abstract in AMA style:
Bardin T, Le L, Nguyen Q, Do A, Le H, Do M, Vu A, Le A, Bui K, Richette P, Resche-Rigon M, Mai T. Risk Factors for Cutaneous Reactions to Allopurinol in Kinh Vietnameses: Results of a Prospective Study in Ho Chi Minh City [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-factors-for-cutaneous-reactions-to-allopurinol-in-kinh-vietnameses-results-of-a-prospective-study-in-ho-chi-minh-city/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-cutaneous-reactions-to-allopurinol-in-kinh-vietnameses-results-of-a-prospective-study-in-ho-chi-minh-city/