Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In October 2017, the Australian Rheumatology Association widely promoted a list of five recommendations on low-value practices to general clinicians and patients as part of the peer-reviewed Evolve initiative. One recommendation in keeping with international guidelines suggested avoiding ordering anti-double-stranded DNA antibodies (dsDNA) in anti-nuclear antibody (ANA) negative patients unless there was a high clinical suspicion of Systemic Lupus Erythematosus (SLE). Inappropriate anti dsDNA antibody ordering confers a substantial and unnecessary cost on health resources.
We sought to compare ANA and anti dsDNA antibody ordering practices in a large tertiary teaching hospital before and after the Evolve recommendations were promoted.
Methods: Results of ANA and dsDNA testing ordered through the institutional laboratory of a large tertiary teaching hospital between 2012-2018 inclusive were captured, as were data paired to these tests including age, gender and the ordering medical specialty. Analysis was performed on results ordered between January 1 and March 31 of each year. Retrospective chart review of ANA negative patients with dsDNA testing ordered was performed to review the indication for testing and the presence of component clinical criteria from the Systemic Lupus International Collaborating Clinics Classification (SLICC) Criteria for Systemic Lupus Erythematosus.
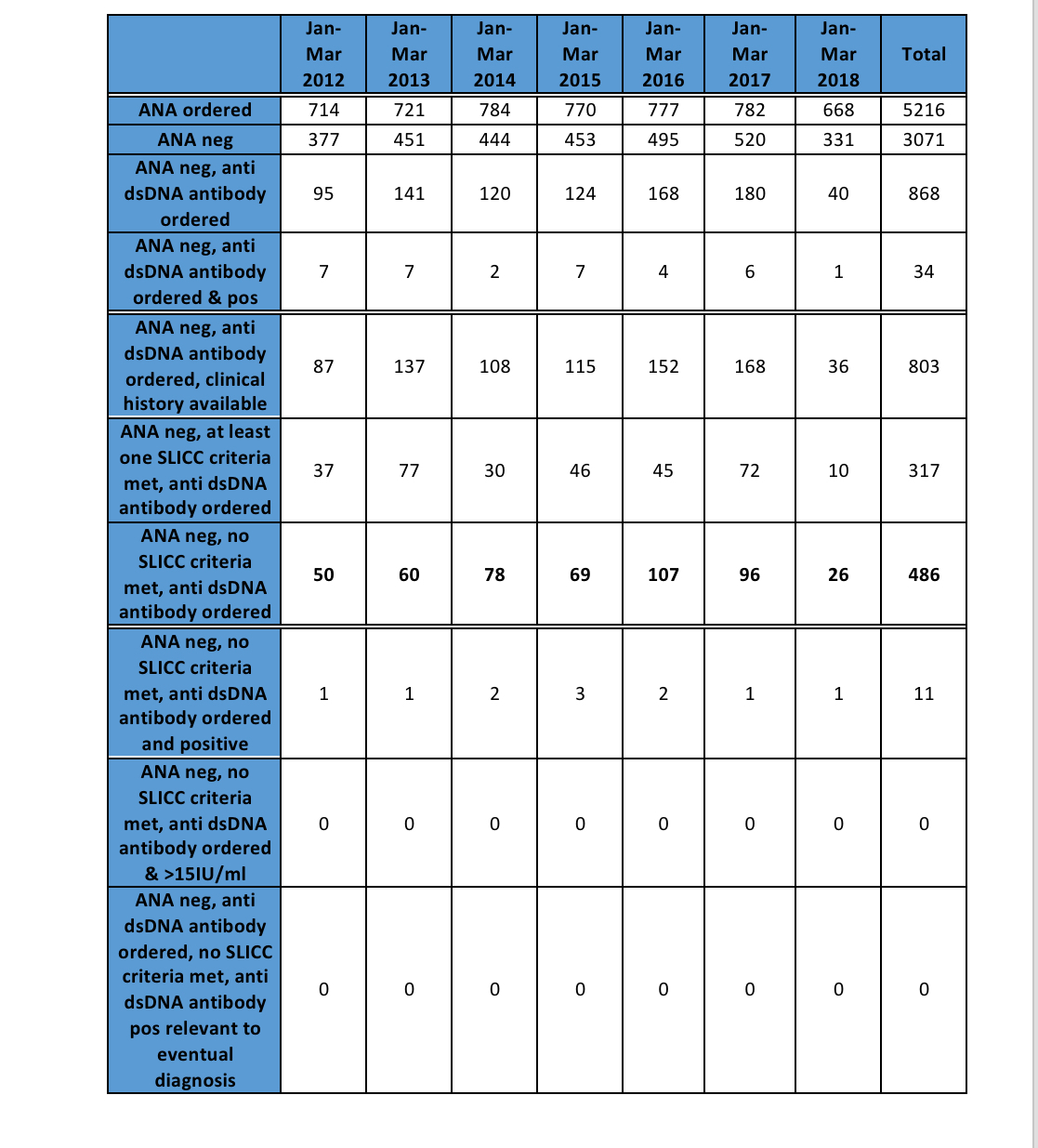
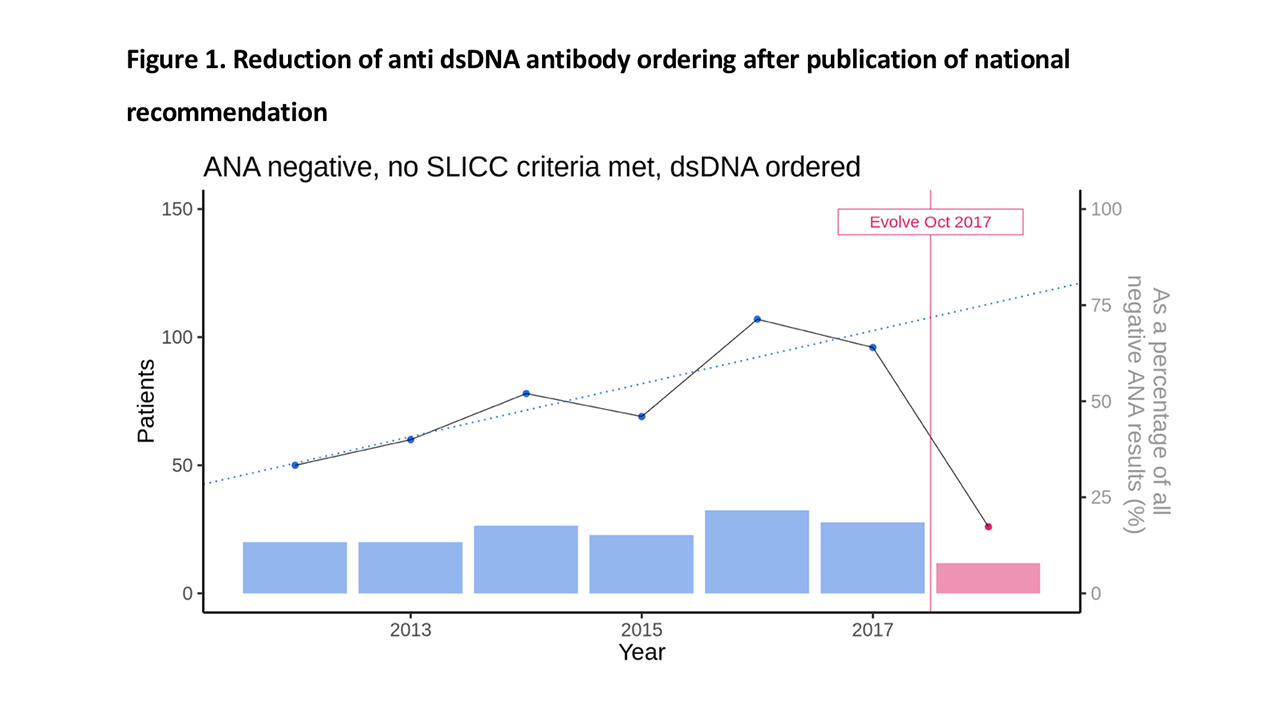
Results: A total of 24,501 ANA tests were performed between January 1, 2012 and August 16, 2018. Of these, 5216 patients had ANA tests ordered between January 1 and March 31 in any year, with 3071 having returned a negative ANA result and 803 of these having also had a dsDNA ordered (Table 1). The majority of these patients had no history of any SLICC clinical criteria (486, 60.5%). Very few ANA negative patients who did not meet SLICC criteria had positive dsDNA (14, 2.8%) and all were low titre. None of these dsDNA results were relevant to the patient’s eventual diagnosis. There was a marked reduction in dsDNA ordering following the promotion of the Evolve recommendations starting October 2017, from 96 patients during January – March 2017 to 28 patients during Januaryy – March 2018 (Figure 1).
Conclusion: A majority of patients who had dsDNA testing ordered despite a negative ANA result had no clinical features of SLE. There has been a marked reduction in the ordering of dsDNA testing in ANA negative
To cite this abstract in AMA style:
Anjara P, Liew D, Yang V, McMaster C, Buchanan R. Frequency of Performing Anti dsDNA Antibody in an ANA Negative Patient with Clinical Suspicion of SLE in a Single Centre Trial Before and After the Publication of National Guideline [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/frequency-of-performing-anti-dsdna-antibody-in-an-ana-negative-patient-with-clinical-suspicion-of-sle-in-a-single-centre-trial-before-and-after-the-publication-of-national-guideline/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/frequency-of-performing-anti-dsdna-antibody-in-an-ana-negative-patient-with-clinical-suspicion-of-sle-in-a-single-centre-trial-before-and-after-the-publication-of-national-guideline/