Session Information
Date: Sunday, November 10, 2019
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Type I Interferons (IFN) play a key role in the pathogenesis and evolution of various autoimmune diseases. Previous studies have demonstrated that the expression of a number of genes regulated by type I IFN, the so-called “IFN signature”, has been related with disease activity and clinical features in systemic lupus erythematosus (SLE) patients and other systemic conditions. However, differences in the degree of activation and gene diversification have been reported among diseases and even at the disease level. To date, a limited number of studies have analysed the IFN signature in antiphospholipid syndrome (APS) setting, both primary (PAPS) and when associated with other autoimmune conditions (secondary APS, SAPS). This study aims to describe the activation and structure of the type I IFN signature among different subsets of APS.
Methods: A total of 116 patients were enrolled, including 19 PAPS patients, 13 SAPS, 75 SLE patients, and 9 antiphospholipid antibodies positive individuals (“aPL carriers”). Thirty-two subjects were also recruited as healthy controls (HC).
IFI44, IFI44L, IFI6, MX1 and IRF4 gene expression was determined in whole blood in the entire cohort. Expression levels were normalized to Z-scores and averaged into a global IFN signature. Differences were measured by Kruskal-Wallis tests and associations among genes were studied by cluster and correspondence analyses. Correlations were plotted by network analyses.
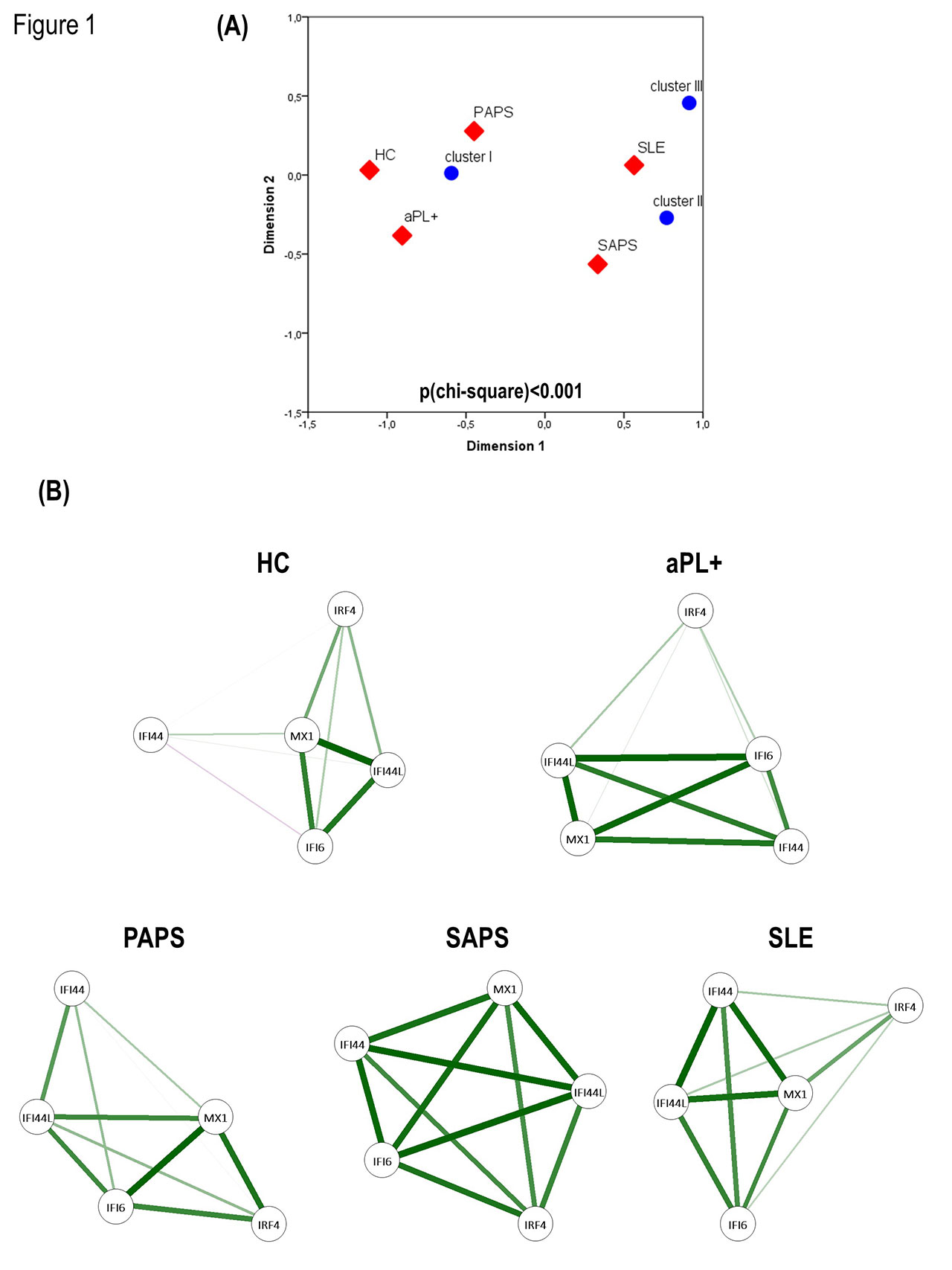
Results: A global activation of the type I IFN signature was observed (HC: -0.44±0.08, aPL carriers: -0.38±0.12, PAPS: -0.31±0.80, SAPS: -0.17±0.39, SLE: 0.09±0.80; p(Kruskal-Wallis)< 0.001).Certain heterogeneity was observed among interferon regulated genes (IRG): MX1 being increased in all patient groups (all p< 0.001), whereas IFI44 was only increased in SLE (p< 0.001) and PAPS (p< 0.001), and both IFI44L and IFI6 were increased in SLE (both, p< 0.001) and a trend was observed in SAPS (p=0.060 and p=0.080). By means of an unsupervised analysis, 3 clusters (I to III) were identified, which correlated with clinical status of the patients by correspondence analysis (p< 0.0001, Figure 1a). Network analyses revealed different structures of the IRGs networks among groups, from weaker networks in HC and aPL carriers to stronger degree of correlations among IRGs in SAPS and SLE, thus pointing to diverse expression programs (Figure 1b). Among APS patients (both SAPS and PAPS), the IFN signature was positively associated with anti-phosphatidylserine/prothrombin antibodiesIgG levels (r=0.478, p=0.003), but no associations were observed for the IgM isotype nor with other autoantibodies specificities (all p >0.050). No associations were observed with traditional cardiovascular risk factors or current treatments (all p >0.050).
Conclusion: APS is associated with a broad type I IFN activation, with a notable heterogeneity within the IFN signature structure among clinical subsets.
Panel A- Clusters analysis, correlated with clinical status of the patients by correspondence analysis -p<0.0001-.
Panel B- Network analyses of the interferon regulated genes networks among groups.
To cite this abstract in AMA style:
Cecchi I, Radin M, Rubini E, Foddai S, Suarez A, Menegatti E, Roccatello D, Sciascia S, Rodriguez Carrio J. Type I Interferon Signature Activation in Antiphospholipid Syndrome: Gene Expression Heterogeneity Among Disease Subsets [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/type-i-interferon-signature-activation-in-antiphospholipid-syndrome-gene-expression-heterogeneity-among-disease-subsets/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/type-i-interferon-signature-activation-in-antiphospholipid-syndrome-gene-expression-heterogeneity-among-disease-subsets/