Session Information
Session Type: ACR Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Behçet’s syndrome is a chronic, relapsing, multi-system inflammatory disorder characterized by recurrent oral ulcers (OU), which can be disabling and substantially impact quality of life. In a phase III study (RELIEF), apremilast (APR) reduced the number and pain of OU in patients with active Behçet’s syndrome over 12 weeks.
Methods: In this phase III, multicenter, placebo (PBO)-controlled study, adult patients with active Behçet’s syndrome (defined by ≥3 OU at randomization or ≥2 OU at screening and at randomization without active major organ involvement) were randomized (1:1) to PBO or APR 30 mg BID for 12 weeks. All patients then received APR treatment through Week 64. The primary endpoint was area under the curve (AUCWk0-12) for total number of OU over 12 weeks, which reflects the number of OU over time and accounts for the recurring-remitting course of OU. The current analysis assessed APR efficacy in the treatment of OU for up to 28 weeks.
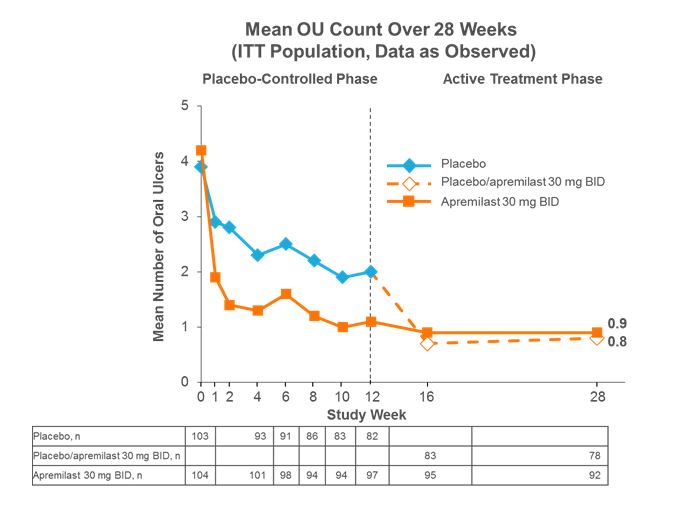
Results: A total of 207 patients were randomized and received ≥1 dose of study medication (PBO: n=103; APR: n=104). At baseline (BL), mean OU counts were 4.2 (APR) and 3.9 (PBO) and mean visual analog scale pain scores were 61.2 (APR) and 60.8 (PBO). The primary endpoint of AUCWk0-12 was achieved; a statistically significantly lower AUCWk0-12 was observed for APR vs. PBO (129.54 vs. 222.14; P<0.0001, multiple imputation). APR treatment resulted in a significantly lower number of OU (P≤0.0015, multiple imputation) and reduction in OU pain (P≤0.0035, mixed-effects model for repeated measures) compared with PBO at every visit from Week 1 through Week 12. APR efficacy was sustained with continued treatment through 28 weeks. At Week 28, 62% of patients achieved complete response of OU, with a 70% relative reduction in OU pain from BL. Patients initially randomized to PBO and switched to APR at Week 12 showed comparable benefits (Figures). At Week 28, 59.0% of patients achieved complete remission of OU, with a 68% relative reduction in OU pain from BL. The incidence of any adverse event (AE) was comparable between APR and PBO during the PBO-controlled period (78.8% and 71.8%, respectively). The most common AEs were diarrhea, nausea, headache, and upper respiratory tract infection; most AEs were mild or moderate in severity. No new safety concerns were identified with up to 28 weeks of APR treatment.
Conclusion: APR demonstrated efficacy in the treatment of OU in patients with active Behçet’s syndrome. Benefits were sustained for up to 28 weeks with continued treatment. APR was generally well tolerated and safety was consistent with the known safety profile of apremilast.
To cite this abstract in AMA style:
Hatemi G, Mahr A, Ishigatsubo Y, Song YW, Melikoglu M, Cheng S, McCue S, Paris M, Chen M, Yazici Y. Efficacy of Apremilast for Oral Ulcers Associated with Active Behçet’s Syndrome over 28 Weeks: Results from a Phase III Study [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-apremilast-for-oral-ulcers-associated-with-active-behcets-syndrome-over-28-weeks-results-from-a-phase-iii-study/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-apremilast-for-oral-ulcers-associated-with-active-behcets-syndrome-over-28-weeks-results-from-a-phase-iii-study/