Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Cutaneous lupus erythematosus (CLE) affects ~75% of SLE patients and has a profound impact on quality of life. While the morbidity from CLE is significant, effective therapeutic options, including topical therapies, are limited. There is growing evidence that the manipulation of the endocannabinoid system has immunomodulatory activity which could be clinically translated to a broad range of cutaneous and systemic inflammatory diseases, although data is limited especially with respect to topical administration. In this study, local application of anandamide (AEA), a highly lipophilic endocannabinoid, was evaluated in MRL/lpr lupus prone mice, which spontaneously develop skin lesions similar to chronic CLE both clinically and histologically. To overcome known issues with respect to topical delivery of cannabinoids, nanoparticle encapsulation was employed.
Methods:
Nanoparticles with 125 nm radius were loaded with 4% AEA (AEA-np) and mixed with coconut oil (25% by weight). Starting at 10 weeks of age, female MRL/lpr mice were treated twice a week with interscapular application of the AEA compound without hair removal. Control groups of mice were treated topically with empty nanoparticles (“control treated”) or received only coconut oil (“untreated”). Mice were regularly scored using a validated modified CLASI tool to assess the effect of AEA on cutaneous disease. At 20 weeks of age, mice were sacrificed and the skin harvested for histological analysis.
Results:
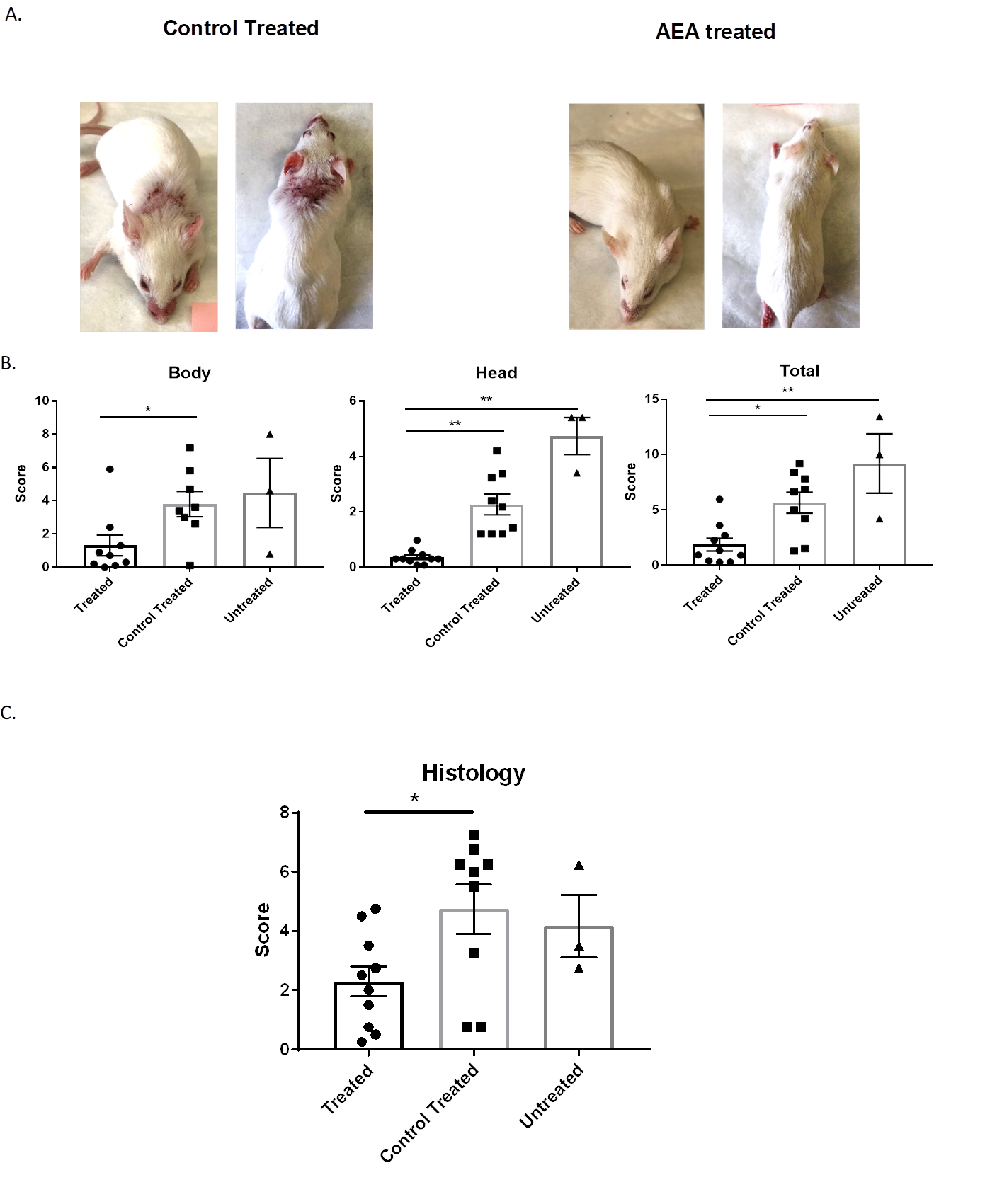
AEA-np treated mice had significantly improved skin lesions. Interestingly, this improvement was noted both where the nanoparticles were directly applied, as well as the face/snout region. At the time of sacrifice, AEA treated mice had significantly less macroscopic lesions (Figure 1A), which were scored blindly (p<0.0001) (Figure 1B). Moreover, significant histologic improvement was seen as well (p<0.05; Figure 1C). No differences were seen in systemic disease parameters, namely circulating anti-dsDNA antibodies and proteinuria levels. Immunofluorescent staining of skin sections to characterize cellular infiltration and mechanistic studies in vitro are in progress.
Conclusion:
Together, these data demonstrate that topical administration with AEA loaded nanoparticles significantly prevents the development of CLE in an established animal model of lupus. Furthermore, this work reinforces and highlights the utility of targeting the endocannabinoid system for autoimmune rheumatic diseases.
Figure 1
To cite this abstract in AMA style:
Chalmers S, Garcia S, Draganski A, Doerner J, Friedman A, Friedman J, Putterman C. Topical Endocannabinoid Administration Protects MRL-Lpr/Lpr Mice from Cutaneous Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/topical-endocannabinoid-administration-protects-mrl-lpr-lpr-mice-from-cutaneous-lupus-erythematosus/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/topical-endocannabinoid-administration-protects-mrl-lpr-lpr-mice-from-cutaneous-lupus-erythematosus/