Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Novel Serum Biomarkers Monitor Disease Progression and Response to Change in Corticosteroid Therapy in Children with Juvenile Dermatomyositis
Background/Purpose: Juvenile Dermatomyositis (JDM) is a complex autoimmune disease with varying responses to therapy. Serum protein biomarkers could monitor therapy and test new drugs. The purpose of this pilot study was to identify corticosteroid-responsive serum biomarkers and determine their correlation with differing Myositis Specific Antibodies (MSA) in JDM.
Methods: The CureJM Juvenile Myositis Registry was searched for children diagnosed with definite/probable JDM who had MSA (by immunoprecipitation), and who also had sera available at three time points: 1) before start of treatment, 2) on therapy based on corticosteroids, and 3) when less clinically active and off corticosteroid therapy. Serum samples from JDM patients meeting these criteria (n=8, mean age = 6.8 ± 2.7 years) were tested for 1,300 proteins (SOMAscan proteomics). Data were benchmarked against data from 12 healthy control children, age 6-10, mean age 8.5±1.7 years. To reduce heteroscedasticity the data was log transformed before statistical analysis. A two sample t test with adjustment for multiple testing was performed to compare protein levels between JDM and controls. Statistical analysis was performed with the R language for statistical computing v3.4.2.
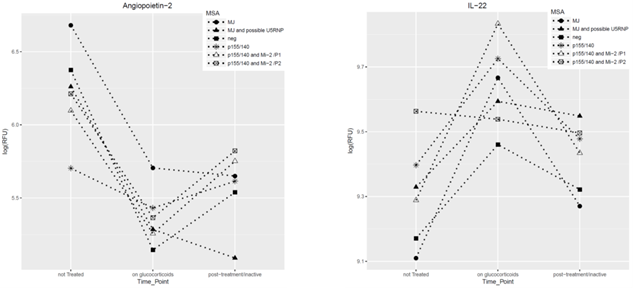
Results: In untreated JDM, preliminary data analysis identified 44 elevated proteins and 79 proteins that were decreased compared to controls—adjusted p value of <0.001. The elevated proteins were primarily immunologic mediators (chemokines, interleukins), confirming previous studies, and included vascular associated proteins such as angiopoietin 2 (novel biomarker), which induces apoptosis of endothelial cells and vascular regression and is an indicator of atherosclerosis. While on prednisone, levels of proteins such as angiopoietin 2, IP10 and myostatin decreased as did levels of CXCL-11, which is regulated by Interferon-beta, a major pro-inflammatory component of JDM. When prednisone was decreased, some of these biomarkers returned to their original levels, prior the treatment, while others stayed low, depending on the MSA of the child. Figure 1 below displays examples of changes in the level of 2 serum biomarkers with therapy.
Conclusion: Specific serum proteins are candidates for consideration as potential biomarkers to monitor response to therapies in children with JDM. Limitations of this pilot study include the low numbers of subjects, which will require validation in additional cohorts.
Figure 1. Two novel JDM serum biomarkers and their longitudinal trajectory with therapy in JDM by MSA. Serum levels of both proteins changed after therapy started and tended to rebound after therapy decreased. The child with MJ and possible U5RNP is the exception; angiopoietin-2 and IL-22 did not return to their original levels after therapy was stopped.
To cite this abstract in AMA style:
Pachman LM, Tawalbeh S, Morgan GA, Marin W, Conklin L, Hathout Y, Hoffman E. Novel Serum Biomarkers Monitor Disease Progression and Response to Change in Corticosteroid Therapy in Children with Juvenile Dermatomyositis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/novel-serum-biomarkers-monitor-disease-progression-and-response-to-change-in-corticosteroid-therapy-in-children-with-juvenile-dermatomyositis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-serum-biomarkers-monitor-disease-progression-and-response-to-change-in-corticosteroid-therapy-in-children-with-juvenile-dermatomyositis/